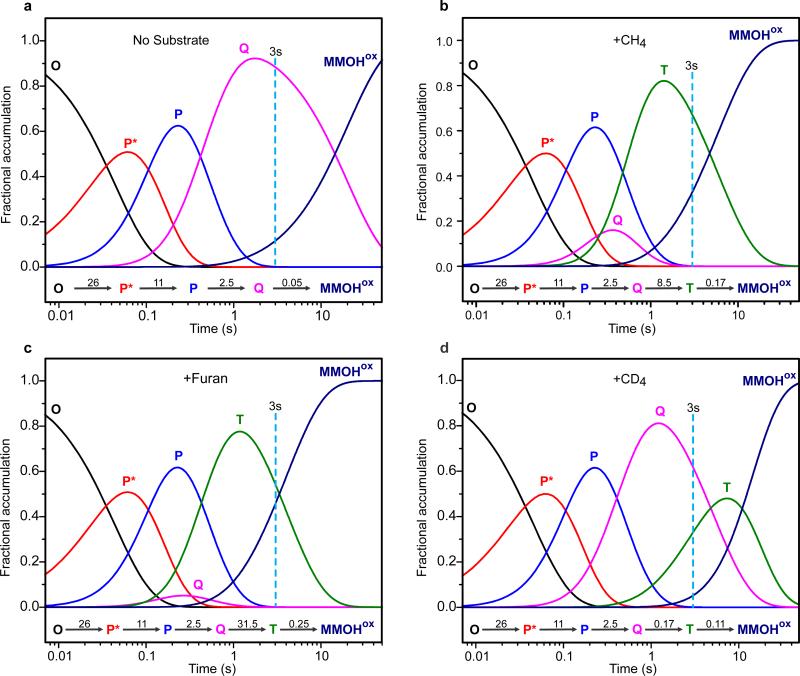
Extended Data Figure 1. Speciation plots of the sMMO reaction with O2.
Plots were computed using known rate constants of the catalytic steps1,8,32,33 for the following conditions. a, No added substrate, b, presence of 0.45 mM CH4, c, presence of 3.5 mM furan and d, presence of 0.45 mM CD4 at pH 7.0, 4 °C. Rate constants used in simulation of individual conditions are shown for each step. All rate constants are first order (s−1) except for the Q to T step, which is first order in both Q and substrate (M−1 s−1) but is given as a pseudo first order constant for the current substrate concentration. The rate constant for the formation of intermediate O (oxygen binding) is unknown, but it is assumed to be fast based upon typical rates for metalloenzymes of the MMO type. It is irreversible because the rate constant of the next step (O → P*) is independent of O2 concentration.