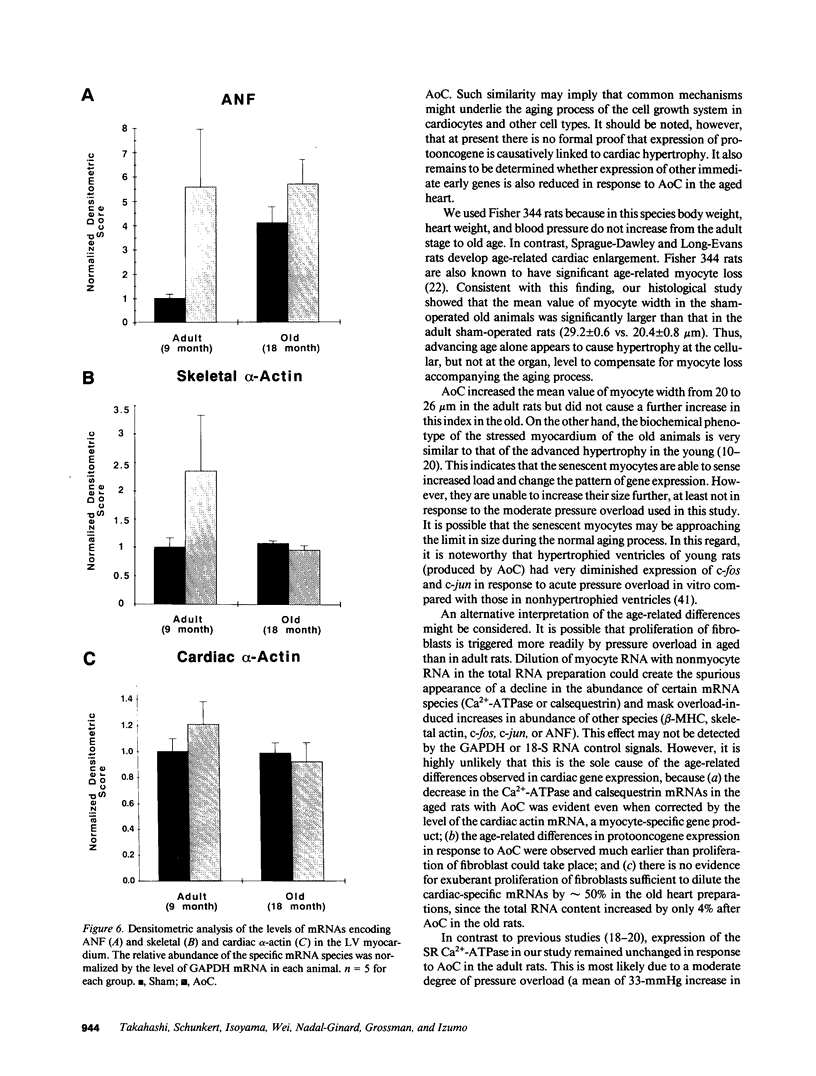
Abstract
Cardiac adaptation to hemodynamic stress involves both quantitative (hypertrophy) and qualitative (pattern of gene expression) changes. Our previous studies have shown that advancing age in the rat is associated with diminished capacity to develop left ventricular hypertrophy in response to either ascending aortic constriction (AoC). In this study, we examined whether the expression of protooncogenes and contractile protein genes in response to AoC differs between adult (9-mo-old) and old (18-mo-old) rats. RNA was isolated from the left ventricles of AoC animals of both age groups subjected to a similar hemodynamic stress. Immediately after AoC, the levels of the ventricular expression of c-fos and c-jun protooncogenes were markedly lower in the old rats than in the adult animals. 5 d after the operation, the ratio of beta- to alpha-myosin heavy chain mRNAs increased significantly after AoC in both age groups. In contrast, AoC was associated with a marked reduction in the levels of mRNAs encoding sarcoplasmic reticulum Ca(2+)-ATPase (by 69%) and cardiac calsequestrin (by 49%) in the old rats but not in the adults. The mRNAs encoding atrial natriuretic factor and skeletal alpha-actin increased in response to AoC only in the adult rats. There were no significant differences in expression of the cardiac alpha-actin mRNA among the experimental groups. These data suggest that (a) the expression of protooncogenes in response to acute pressure overload is significantly reduced in the aged rats and (b) the pattern of expression of the contractile protein gene in response to AoC in the old rats differs qualitatively as well as quantitatively from that in younger animals. These age-related differences may play a role in the higher frequency of heart failure in the aged during hemodynamic stress.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Anversa P., Hiler B., Ricci R., Guideri G., Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardiol. 1986 Dec;8(6):1441–1448. doi: 10.1016/s0735-1097(86)80321-7. [DOI] [PubMed] [Google Scholar]
- Anversa P., Puntillo E., Nikitin P., Olivetti G., Capasso J. M., Sonnenblick E. H. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol. 1989 May;256(5 Pt 2):H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- Day M. L., Schwartz D., Wiegand R. C., Stockman P. T., Brunnert S. R., Tolunay H. E., Currie M. G., Standaert D. G., Needleman P. Ventricular atriopeptin. Unmasking of messenger RNA and peptide synthesis by hypertrophy or dexamethasone. Hypertension. 1987 May;9(5):485–491. doi: 10.1161/01.hyp.9.5.485. [DOI] [PubMed] [Google Scholar]
- Effron M. B., Bhatnagar G. M., Spurgeon H. A., Ruaño-Arroyo G., Lakatta E. G. Changes in myosin isoenzymes, ATPase activity, and contraction duration in rat cardiac muscle with aging can be modulated by thyroxine. Circ Res. 1987 Feb;60(2):238–245. doi: 10.1161/01.res.60.2.238. [DOI] [PubMed] [Google Scholar]
- Farrar R. P., Starnes J. W., Cartee G. D., Oh P. Y., Sweeney H. L. Effects of exercise on cardiac myosin isozyme composition during the aging process. J Appl Physiol (1985) 1988 Feb;64(2):880–883. doi: 10.1152/jappl.1988.64.2.880. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich J. P., Lakatta E. G., Beard E., Spurgeon H. A., Weisfeldt M. L., Gerstenblith G. Studies of sarcoplasmic reticulum function and contraction duration in young adult and aged rat myocardium. J Mol Cell Cardiol. 1978 May;10(5):427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980 Oct;69(4):576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- Isoyama S., Grossman W., Wei J. Y. Effect of age on myocardial adaptation to volume overload in the rat. J Clin Invest. 1988 Jun;81(6):1850–1857. doi: 10.1172/JCI113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S., Wei J. Y., Izumo S., Fort P., Schoen F. J., Grossman W. Effect of age on the development of cardiac hypertrophy produced by aortic constriction in the rat. Circ Res. 1987 Sep;61(3):337–345. doi: 10.1161/01.res.61.3.337. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med. 1990 Jan 11;322(2):100–110. doi: 10.1056/NEJM199001113220206. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Shibazaki Y., Takaku F., Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989 Apr;83(4):1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E. G., Yin F. C. Myocardial aging: functional alterations and related cellular mechanisms. Am J Physiol. 1982 Jun;242(6):H927–H941. doi: 10.1152/ajpheart.1982.242.6.H927. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Maciel L. M., Polikar R., Rohrer D., Popovich B. K., Dillmann W. H. Age-induced decreases in the messenger RNA coding for the sarcoplasmic reticulum Ca2(+)-ATPase of the rat heart. Circ Res. 1990 Jul;67(1):230–234. doi: 10.1161/01.res.67.1.230. [DOI] [PubMed] [Google Scholar]
- McCafferty W. B., Edington D. W. Skeletal muscle and organ weights of aged and trained male rats. Gerontologia. 1974;20(1):44–50. doi: 10.1159/000211997. [DOI] [PubMed] [Google Scholar]
- Mendez R. E., Pfeffer J. M., Ortola F. V., Bloch K. D., Anderson S., Seidman J. G., Brenner B. M. Atrial natriuretic peptide transcription, storage, and release in rats with myocardial infarction. Am J Physiol. 1987 Dec;253(6 Pt 2):H1449–H1455. doi: 10.1152/ajpheart.1987.253.6.H1449. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Samuel J. L., Michel J. B., Zongazo M. A., de la Bastie D., Lompre A. M., Wisnewsky C., Rappaport L., Levy B., Schwartz K. Atrial natriuretic factor gene expression in rat ventricle during experimental hypertension. Am J Physiol. 1989 Sep;257(3 Pt 2):H979–H987. doi: 10.1152/ajpheart.1989.257.3.H979. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991 Jan;83(1):13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Nagai R., Zarain-Herzberg A., Brandl C. J., Fujii J., Tada M., MacLennan D. H., Alpert N. R., Periasamy M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2966–2970. doi: 10.1073/pnas.86.8.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N. Comparison of ATP-dependent calcium transport and calcium-activated ATPase activities of cardiac sarcoplasmic reticulum and sarcolemma from rats of various ages. Mech Ageing Dev. 1987 Apr;38(2):127–143. doi: 10.1016/0047-6374(87)90073-x. [DOI] [PubMed] [Google Scholar]
- Paulsson Y., Bywater M., Pfeifer-Ohlsson S., Ohlsson R., Nilsson S., Heldin C. H., Westermark B., Betsholtz C. Growth factors induce early pre-replicative changes in senescent human fibroblasts. EMBO J. 1986 Sep;5(9):2157–2162. doi: 10.1002/j.1460-2075.1986.tb04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. D., Parker T. G. Cardiac myocytes as targets for the action of peptide growth factors. Circulation. 1990 May;81(5):1443–1456. doi: 10.1161/01.cir.81.5.1443. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Jahn L., Izumo S., Apstein C. S., Lorell B. H. Localization and regulation of c-fos and c-jun protooncogene induction by systolic wall stress in normal and hypertrophied rat hearts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11480–11484. doi: 10.1073/pnas.88.24.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Scott B. T., Simmerman H. K., Collins J. H., Nadal-Ginard B., Jones L. R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Smith C. W., Izumo S., Grant J. W., Endo T., Andreadis A., Nadal-Ginard B. The expression of sarcomeric muscle-specific contractile protein genes in BC3H1 cells: BC3H1 cells resemble skeletal myoblasts that are defective for commitment to terminal differentiation. J Cell Biol. 1989 May;108(5):1799–1806. doi: 10.1083/jcb.108.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wei J. Y., Gersh B. J. Heart disease in the elderly. Curr Probl Cardiol. 1987 Jan;12(1):1–65. [PubMed] [Google Scholar]
- de la Bastie D., Levitsky D., Rappaport L., Mercadier J. J., Marotte F., Wisnewsky C., Brovkovich V., Schwartz K., Lompré A. M. Function of the sarcoplasmic reticulum and expression of its Ca2(+)-ATPase gene in pressure overload-induced cardiac hypertrophy in the rat. Circ Res. 1990 Feb;66(2):554–564. doi: 10.1161/01.res.66.2.554. [DOI] [PubMed] [Google Scholar]