Abstract
Purpose
To observe changes in semen parameters, sperm DNA integrity, chromatin condensation and cysteinyl aspartate-spicific proteinases (Caspase-3) in adult healthy men after scrotal heat stress (SHS).
Methods
The scrotums of 19 healthy male volunteers were exposed to the condition of 40–43 °C SHS belt warming 40 min each day for successive 2 days per week. The course of SHS was continuously 3 months. Routine semen analysis, hypo-osmotic swelling (HOS) test, eosin Y (EY) staining sperm HOS and chromatin dispersion (HOS/SCD) test, HOS and aniline blue (HOS/AB) staining test were carried out before, during and after SHS. The activated Caspase 3 levels of spermatozoa were determined with a microtiter plate reader.
Results
The mean parameters of sperm concentration, motility and normal morphological sperm were significantly decreased in groups with sperm being collected during SHS 1, 2 and 3 months when compared with those in groups of pre-SHS (P < 0.01). Statistically significant differences of sperm DNA fragmentation, normal sperm membrane and vitality, and Caspase-3 activity were observed between the groups of before SHS and after SHS 3 months and the groups of during SHS 1, 2 and 3 months (P < 0.001). Three months the SHS stopped, various parameters recovered to the level before SHS. Abnormal sperm with HOS/AB and HOS/SCD showed a negatively significant correlation with normal sperm by HOS/EY test, and WBC in semen showed a positively significant correlation with Caspase-3 activity. The percentage of abnormal sperm by using the test of HOS/SCD showed a positively significant correlation with that of HOS/AB.
Conclusions
The continuously constant SHS can impact the semen quality, sperm DNA integrity, chromatin condensation and Caspase-3, and the combination of HOS plus AB test may simultaneously determine the integrity of membrane and chromatin condensation at the same spermatozoon.
Keywords: Sperm, Scrotal heating, Sperm chromatin condensation, Caspase-3, Aniline blue staining
Introduction
Spermatogenesis is a temperature-dependent process. For normal spermatogenesis, of most mammals, the temperature in the scrotum is 2–8 °C lower than that of the core body temperature [1, 2]. Elevation of scrotal temperature has been known as a cause of male infertility but the exact mechanism leading to impaired spermatogenesis is unknown [3]. Recent study suggested that scrotal hyperthermia by exposure to sauna can induce a significant alteration of spermatogenesis [4]. There may be a variety of mechanisms of testis that are triggered on exposure to stress, including DNA repair, heat shock response, oxidative stress response, and apoptosis and cell death [5]. Scrotal hypothermia can cause a reversible disruption of the blood-testis barrier (BTB) [6]. Apoptosis is a mode of programmed cellular death based on a genetic mechanism that induces a series of cellular, morphological and biochemical alterations. Mature sperm cells have been reported to express distinct markers of apoptosis-related cell damage [7, 8].
Some studies have reported that localized scrotal heating of animals is associated with detection of DNA damage in sperm and reduced sperm counts [9–11]. Spermatogenesis is disrupted by scrotal exposure to environmental temperatures over normal physiological temperature. Scrotal heat stress can compromise the DNA integrity of spermatozoa. Wang et al. observed semen parameters, testicular histology, and germ cell apoptosis after scrotal heating plus testosterone, and found that apoptosis with heat and testosterone treatment occured mainly in the round spermatids and pachytene spermatocytes [11]. When DNA damage was detected in sperm recovered from the epididymes within hours of heating this was taken as evidence that epididymal sperm are still susceptible to heat-induced DNA damage even though the DNA is tightly packaged with protamines. The hypo-osmotic swelling (HOS) test and the Eosin Y (EY) staining can evaluate sperm motality and detect the integrity of the tail membrane of sperm [12–14]. Sperm DNA fragmentation or sperm chromatin status can be evaluated in a variety of ways, including the sperm chromatin structure assay (SCSA) [12–15], terminal deoxynucleotidyl transferase-mediated d UDP nick-end labeling (TUNEL) [15], single cell gel electrophoresis (COMET) assay [16], the sperm chromatin dispersion (SCD) test and aniline blue (AB) staining [17–20]. The degree of condensation can be shown with the aid of acidic aniline blue (AB) staining, which is able to discriminate between lysine-rich histones and arginine- and cysteine-rich protamines. However, the vitality and the integrity of the sperm membrane could not be determined using these tests [12–14].
Caspase (Cysteine-requiring Aspartate Protease) is a protease family, which plays an important role in apoptosis process. Caspase-3 is a key enzyme in apoptosis process. In all the caspases, Caspase-3 is the most studied in mammalian cells. Activated caspase-3 and loss of the integrity of the DNA fragmentation are other markers of terminal apoptosis expressed by a varying proportion of ejaculated sperm [21–23]. It has been hypothesized that sperm cell death is associated with male infertility. However, the exact mechanisms of its involvement and elevated intra-testicular temperature remain to be elucidated.
The objective of this study was to evaluate whether semen parameters, sperm DNA integrity, chromatin condensation and activity of sperm Caspase-3 are altered in 19 adult healthy men after scrotal heat stress (SHS) and whether methods of determining sperm DNA integrity, sperm chromatin and caspase-3 have correlations.
Materials and methods
Scrotal heat stress (SHS) devise
SHS consisted water bag, electric heating system, timer and temperature controller, and temperature probe. The water bag was attached to the underpants by 4 belts. The input and output Voltage of the SHS’s electric heating system were 200 V and 12 V, respectively. The time was 1–60 min. The temperature ranged from 40 to 43 °C. A thermal probe was attached to the underpants and the temperature was shown on the electronic monitor.
Equipment and reagents
Computer-aided sperm analysis (CASA) machines (WLJY-9000, Weili, Bejing, China) were used for kinematic analysis of spermatozoa. A Leica microscopy (DM4000B; Leica, Wetzlar, Germany) and a phase-contrast microscopy (IX51, Olympus, Japan) were used to observe sperm and cells. Sperm Morphology Staining Kit (Diff Quik, YZB-0058-2011) and Aniline Blue Kit were purchased from Hua Kang (YZB-0058-2012, Hua Kang Co. LTD. Shenzhen, China). The Caspase-3 Activity was determined by the Microplate plate reader (ELISA-reader, SMP500-18410-EMKX VersaMax, Molecular Devices Corporation, USA). The Caspase-3 Activity Assay Kit was purchased from Bioengneering Institute of Nanjing Jiancheng (Nanjing, Jiangsu, China).
Subjects
Between February 2012 and May 2014, 19 healthy adult male volunteers, who already had previously fathered at least one child, were recruited into our study. The mean age of them was 34.75 ± 2.6 years (range, 28–40 years). Prior to this study, subjects were informed of the investigations and provided consent. This study was reviewed by the ethics committee of Shandong Provincial Institute of Science and Technology for Family Planning in China. Each of the 19 subjects had provided semen samples for this study. The approval of the institutional research ethics committee and signed written consent of every subject included in the study were obtained. A detailed medical history was taken and physical examination was performed. Subjects currently on any medication or antioxidant supplementation were not included. Physical examination of subjects before, during and after the SHS included the secondary sexual character, scrotum, penis, spermatic cord, vas deferens, testis and epididymis. In addition, Subjects were also asked about their sex life and with or without erectile dysfunction, anejaculation or other symptoms. The identification information of each subject in this study was kept confidential and was protected from the public.
Volunteers were chosen for the experiments of wearing 40–43 °C SHS for 40 min, 2 days per week for three consecutive months. Semen was collected at 2 and 1 weeks before, and 1, 2 and 3 months during heat treatment and 1, 2 and 3 months after the last SHS.
Routine semen analysis
Semen samples from the 19 healthy subjects were collected by masturbation and ejaculated into sterile glass cups after 3 to 5 days of abstinence before being were analyzed on-site for both macroscopic and microscopic characteristics within 1 h of collection. Information regarding the semen source was withheld from the technologists who performed the semen analyses. Spermogram values, sperm count, percentage of total and progressive motility were determined using a Hamilton Thorn Computer Assisted Sperm Analysis (CASA) system. Density count of leukocytes in seminal plasma was referred to peroxidase dyeing method, recommended by the World Health Orgnization criteria (WHO). Sperm morphology was assessed using the strict (WHO) after Diff-Quik staining [24, 25].
Hypo-osmotic swelling (HOS) test and eosin Y (EY) test and sperm chromatin dispersion (SCD) test and hypo-osmotic swelling (HOS) test
HOS test and eosin Y (EY) staining (HOS/EY) were performed to determine sperm vitality. HOST/EY test was performed as previously reported [9–11]. Four patterns of sperm from HOS/EY test were classified as: 1) Sperm with tail swollen and head unstained was characterized as “normal HOS/EY”. 2) Sperm with unswollen tail and unstained head, or with swollen tail and stained (red) head, or with unswollen tail and stained head was “abnormal HOS/EY”.
After the HOS test had been performed, the SCD test was carried out immediately to evaluate the sperm DNA fragmentation of the 28 subjects. The HOS/SCD methodology has been described previously [11–15].
A total of 500 spermatozoa were evaluated manually on each slide under bright-field optics and oil immersion at ×1000 magnification (DM4000B; Leica, Wetzlar, Germany) [11–15]. The patterns observed for the HOS/SCD test were classified as follows: pattern 1 (A), spermatozoa with large DNA dispersion halos or medium-sized halos and swollen tails (DNA integrity and membrane intact); pattern 2 (B), spermatozoa with large DNA dispersion halos or medium-sized halos and unswollen tails (DNA integrity and membrane damaged); pattern 3 (C), spermatozoa with small-sized halos or the absence of a halo and swollen tails (DNA integrity damaged and membrane intact); and pattern 4(D), spermatozoa with small-sized halos or the absence of a halo and unswollen tails (DNA integrity and membrane damaged).
Aniline blue (AB) staining and HOS
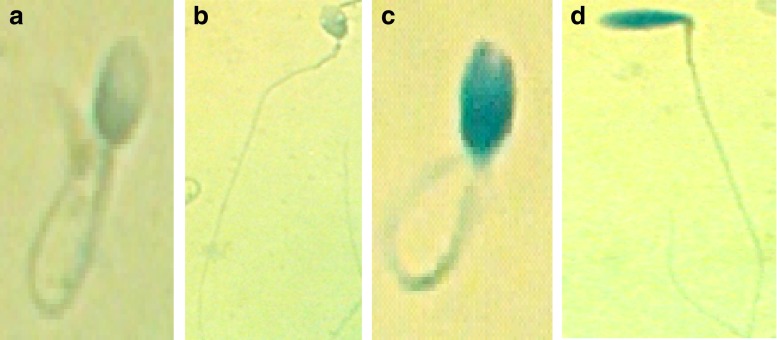
After the HOS test had been performed, the AB test was carried out immediately to evaluate the sperm chromatin condensation of the 28 subjects. A total of 1 mL of the semen sample (mixed with hypoosmotic medium) from the HOS test was centrifuged at 500 g for 5 min, and then the supernatant was discarded and the sperm pellet was resuspended in 0.1 mL of a hypo-osmotic solution. To perform AB staining, sperms were stained with AB as described in a previous report [26, 27]. The slides were prepared by smearing 10 μL of each HOS semen sample. The slides were air-dried and then fixed with a solution of methanol (Hua Kang Co. LTD. Shenzhen, China) in 0.2 M phosphate buffer (pH = 7.2) for 1.5 min at room temperature. Slides were then stained with 5 % aqueous aniline blue solution mixed with 4 % acetic acid (pH = 3.5) for 5 min. This was followed by rinsing and air drying of the slides. For each stained smear, 200 spermatozoa were evaluated with light microscope in oil immersion magnification (100 × objective) (DM4000B; Leica, Wetzlar, Germany). Spermatozoa with unstained (no or light aniline blue-stained) nuclei are considered as normal (clear, mature chromatin) while those blue stained (intermediate and dark aniline blue-stained) were abnormal (immature chromatin) (Fig. 1). Results were expressed as the percentage of nuclear unstained and stained sperm. An ejaculate with a rate of blue-stained nucleus sperm less than 20 % was considered normal. (See Fig. 2.)
Fig. 1.
The hypo-osmotic swelling test aniline blue (AB) staining (HOS/AB) of the same sperm. Patterns observed for the HOS/AB test: a AB1, sperm unstained and swollen tails; b AB2, sperm unstained and unswollen; c AB3, sperm darkly stained swollen tails; d AB4, sperm darkly stained and unswollen
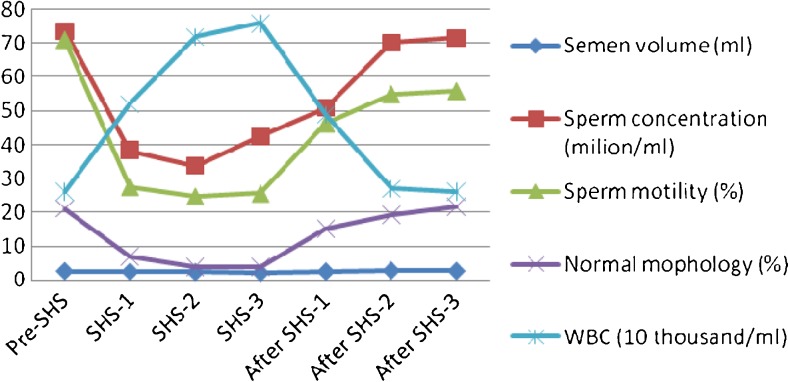
Fig. 2.
Changes in sperm concentration, progressive motility, morphology and WBC before, during and after SHS in 19 men
The patterns observed for the HOS/AB test were classified as follows (mature sperm, Fig. 1): AB1, Mature sperm, having completed histone transition protein–protamine replacement, stained lightly or unstained and swollen tails, membrane intact and no chromatin defects; AB2, spermatozoa with lightly or unstained head and unswollen tails (membrane damaged and no chromatin defects); AB3, spermatozoa with intermediate or dark aniline blue-stained head and swollen tails (chromatin defects and membrane intact); and AB4, spermatozoa with intermediate or dark aniline blue-stained head and unswollen tails (chromatin defects and membrane damaged, severely immature sperm).
Caspase-3 activity
Caspase-3 activity was detected with assay kit of the manufacturer’s protocol (Nanjing Jiancheng, China). The number of 3 ~ 5 × 106 sperms from the semen samples were collected. After centrifugation (2 000 rpm, 5 min), the supernatant was carefully removed, making sure that no cells are removed by suction, the pellet was washed with 300 μl PBS once. The previous process was repeated once before adding lysis buffer to the pellet according to the ratio of 50 μl lysis buffer / 2 million cells. Pellet was resuspended and cleaved 30 min in the ice bath with shaking 3 ~ 4 times, 10 s each time, or freezing and thawing 2 ~ 3 times. Then the sperm samples were centrifuged for 10 ~ 15 min at 4 °C (12 000 rpm). The supernatant (containing protein cleavage) was transfered to fresh tubes and put on ice and added 0.5 μl DTT to per 50 μl lysate, incubated for 1 h. Then 5 μl of DEVD-pNA (Asp-Glu-Val-Asp-p- nitroanilide) was added, incubated at 37 °C for 4 h. The activated Caspase 3 levels were determined with a microtiter plate reader, which can detect the absorbance of 400- or 405-nm. A negative control (sample with 50 μl PBS) and a positive control (sample treated with 10 μM H2O2 for 1 h at 37 °C) were used in all experiments.
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) using the SPSS 13.0 package software (SPSS Inc, Chicago, IL, USA). Differences between groups were verified by one way ANOVA. All the data were presented as mean ± SD. For results of HOS/EY and HOS/AB or HOS/SCD and HOS/EY, Paired-Samples t test was used. Two-tailed Pearson correlation test was used to assess the correlations between groups. A significant statistical difference was accepted when the P value was <0.05.
Results
Changes in semen paramenters before, during and after SHS
Table 1 shows the changes in the sperm concentration, motility (progressive and non-progressive), morphologically normal sperm and white blood cells (WBC) in semen, before, during and after SHS in 19 subjects. Before the SHS experiment, the range of sperm concentration was 29.49–150.67 × 106/ml, the motility rate (progressive and non-progressive) was 39–86 %, and the rate of the normal morphological sperm was 13–34 %. During the SHS 1, 2 and 3 months, the parameters were significantly altered: sperm concentration less than 15 × 106/ml in 6, 7 and 5 of 19 subjects (31.6 %, 36.8 % and 26.3 %. one dropped to 0 during SHS 1); sperm motility rate less than 40 % in 13, 15 and 13 of 19 subjects (68.4 %, 79.0 % and 68.4 %); morphologically normal sperm rate less than 4 % in 5, 10 and 11 of 19 subjects (26.3 %, 52.6 % and 57.9 %), respectively. Significant differences were observed in parameters of sperm concentration, motility, normal morphology and WBC between the pre-SHS group and the group during the SHS 1, 2 and 3 months (P < 0.001). There was no significant difference in the semen volume between the SHS 1- and 2-month group and the pre-SHS group (P > 0.05). The low semen volume observed in the pre-SHS group and the 3-moth SHS group (P = 0.043, and P = 0.02). After 3 months of recovery (stopped SHS), the semen parameters (except sperm motility) gradually returned to normal levels.
Table 1.
Analysis of variances of sperm density, progressive motility, morphology and WBC before, during and after SHS in 19 men using One Way ANOVA
| Pre-SHS (n = 19) | SHS 1 M (n = 19) | SHS 2 M (n = 19) | SHS 3 M (n = 19) | After SHS 1 M (n = 19) | After SHS 2 M (n = 16) | After SHS 3 M (n = 14) | F value | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Semen volume (ml) | 2.59 ± 0.45 | 2.44 ± 0.68 | 2.32 ± 0.48 | 2.11 ± 0.52 | 2.47 ± 0.56 | 2.76 ± 0.97 | 2.64 ± 0.69 | 2.090 | 0.059 |
| Sperm concentration (×106/ml) | 73.62 ± 36.71 | 38.33 ± 24.24 | 33.79 ± 24.05 | 42.56 ± 28.99 | 50.94 ± 30.61 | 70.22 ± 49.10 | 71.7 ± 48.99 | 4.637 | 0.000 |
| Motility (a + b + c %) | 71.0 ± 14.7 | 27.7 ± 20.7 | 24.8 ± 21.7 | 25.7 ± 20.3 | 46.3 ± 22.5 | 55.0 ± 25.3 | 55.9 ± 22.0 | 11.710 | 0.000 |
| Normal Morphology (%) | 21. 1 ± 6.0 | 6.9 ± 4.7 | 3.9 ± 3.8 | 3.8 ± 3.1 | 15.1 ± 3.7 | 19.3 ± 6.1 | 21.7 ± 6.6 | 37.045 | 0.000 |
| WBC (×106/ml) | 0.26 ± 0.14 | 0.52 ± 0.19 | 0.72 ± 0.23 | 0.76 ± 0.31 | 0.49 ± 0.26 | 0.27 ± 0.15 | 0.26 ± 0.14 | 17.356 | 0.000 |
Results of aniline blue (AB) staining, HOS (HOS/AB) and the sperm HOS/SCD test
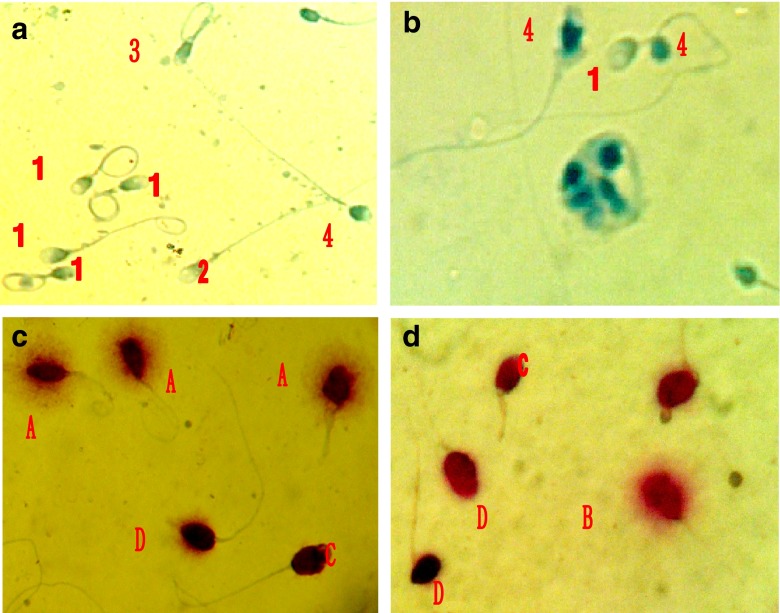
The average percentages of AB1, AB2, AB3, and AB4 sperm from the HOS/AB test, unstained and stained sperm with AB, A, B, C and D sperm with HOS/SCD and swollen and unswollen sperm with HOS/EY for 28 subjects are shown in Table 2. There were no significant decreases between the sum percentages of AB1 plus AB2 (79.3 % + 4.1 % = 83.4 %) and the percentage of unstained (normal) sperm with AB staining alone (83.2 %, P > 0.05), and between the percentages of AB3 and AB4 (abnormal, 4.4 % + 12.2 % = 16.6 %) and the percentage of stained (abnormal) sperm with AB staining alone (16.8 %, P > 0.05), and between the percentages of AB3 and AB1 (4.4 % + 79.3 % = 83.7 %) and the percentage of swollen sperm (84.5 %) with HOS/EY test, and between the percentages of A and C (81.1 % + 3.9 % = 85.0 %) with HOS/SCD test and the percentage of swollen (normal) sperm with HOS/EY test (84.5 %, P > 0.05), and between the percentages of B and D (4.8 % + 10.2 % = 15.0 %) with HOS/SCD test and the percentage of unswollen (abnormal) sperm with HOS/EY test (15.5 %, P > 0.05). The sum of percentages of AB2, AB3 and AB4 sperm (4.1 % + 4.4 % + 12.2 % = 20.7 %) with HOS/AB was higher than that of stained sperm (16.8 %) with AB alone (P < 0.05) and unswollen sperm (abnormal, 15.5 %) with HOS/EY test (P < 0.05), and the sum of percentages of B, C and D (4.8 % + 3.9 % + 10.2 % = 18.9 %) with HOS/SCD was higher than that of unswollen sperm (15.5 %) with HOS/EY test (P < 0.05). Smears from the Diff-Quik staining, HOS/AB, HOS/SCD and the HOS/EY test are shown in Fig. 3.
Table 2.
Patterns of the AB, HOS/EY, HOS/AB and HOS/SCD test in 19 subjects before SHS
| AB (%) | HOS/EY (%) | HOS/AB (%) | HOS/SCD | n |
|---|---|---|---|---|
| Unstained (normal) 83.2 ± 5.6a | Swollen (normal) 84.6 ± 4.1c d | AB1 (normal) 79.3 ± 5.7 | A 81.1 ± 5.7 | 19 |
| AB2 (abnormal) 4.1 ± 1.2 | B 4.8 ± 1.3 | 19 | ||
| AB3 (abnormal) 4.4 ± 1.2 | C 3.9 ± 1.2 | 19 | ||
| Stained (abnormal) 16.8 ± 2.5b | Unswollen (abnormal) 15.4 ± 2.6b | AB4 (abnormal) 12.2 ± 2.3 | D 10.2 ± 2.1 | 19 |
HOS/AB and HOS/EY were observed for the same sperm. AB was only aniline blue for spermatozoa staining
a P < 0.05, Paired-Samples t test, vs AB1
b P < 0.01, Paired-Samples t test, vs AB4 and D of HOS/SCD
c P < 0.01, Paired-Samples t test, vs AB1
d P < 0.05, Paired-Samples t test, vs A of HOS/SCD
Fig. 3.
Sperm morphology, membrane, DNA and chromatin status before and after SHS. a HOS/AB test before SHS, 1 = AB1, 2 = AB2, 3 = AB3 and 4 = AB4 sperm; b HOS/AB test after SHS, 1 = AB1 and 4 = AB4 sperm and macrophage. c HOS/SCD test before SHS, shows A, C and D sperm. d HOS/SCD test after SHS, shows B, C and D sperm
Results of abnormal sperm DNA fragmentation, chromatin condensation, sperm membrane integrity and Caspase 3 activity
The percentage of sperm DNA fragmentation with HOS/SCD, abnormal sperm chromatin condensation with HOS/AB, normal sperm membrane and vitality with HOS/EY, as well as Caspase-3 activity was compared before, during and after the use of SHS in 19 subjects (Table 3). Statistically significant differences of sperm DNA fragmentation, normal sperm membrane and vitality, and Caspase-3 activity were observed between the groups of before SHS and after SHS 3 months and the groups of during SHS 1, 2 and 3 months (P < 0.001). The prevalence of abnormal sperm DNA, and abnormal sperm chromatin condensation, and normal sperm membrane and vitality, and Caspase 3 activity did not show any statistically significant difference between the groups of before SHS and after SHS 3 months (P > 0.05).
Table 3.
Compared analysis of sperm DNA fragmentation, sperm chromatin condensation, the hypoosmotic swelling as well as Caspase 3 activity before, during and after SHS in 19 men using One Way ANOVA
| Pre-SHS (n = 19) | SHS 1 M (n = 19) | SHS 2 M (n = 19) | SHS 3 M (n = 19) | After SHS 1 M (n = 19) | After SHS 2 M (n = 16) | After SHS 3 M (n = 14) | F value | P value | |
|---|---|---|---|---|---|---|---|---|---|
| HOS/SCD | 17.7 ± 3.6 | 70.7 ± 22.3 | 81.3 ± 21.5 | 77.9 ± 23.9 | 23.2 ± 10.0 | 17.9 ± 4.0 | 18.9 ± 6.6 | 52.969 | 0.000 |
| HOS/AB abnormal sperm (%) | 18.9 ± 2.6 | 79.33 ± 22.3 | 83.6 ± 22.1 | 81.4 ± 21.8 | 32.1 ± 9.61 | 23.5 ± 6.6 | 21.6 ± 5.7 | 74.238 | 0.000 |
| HOS/EY normal sperm (%) | 84.6 ± 4.1 | 27.7 ± 20.7 | 24.8 ± 21.7 | 25.7 ± 20.3 | 46.3 ± 22.5 | 80.5 ± 10.1 | 83.7 ± 8.5 | 87.477 | 0.000 |
| Caspase 3 Activity (U/106 sperms) | 1.96 ± 0.91 | 3.08 ± 1.55 | 3.51 ± 1.14 | 3.54 ± 0.72 | 2.22 ± 0.76 | 1.93 ± 0.94 | 1.60 ± 0.94 | 10.775 | 0.000 |
Correlation among sperm parameters of the conventional semen analysis, sperm DNA fragmentation, and abnormal sperm chromatin condensation, and normal sperm membrane and vitality, and Caspase 3 activity
A total of 125 semen samples were collected in 19 subjects before, during 1, 2 and 3 months, and after 1, 2 and 3 months of SHS. Three samples in group after 2 months SHS and five samples in group after 3 months SHS were not collected. Sperm concentration, motility, normal morphology, and normal sperm by HOS/EY test were negatively correlated with the percentage of abnormal sperm by using the test of HOS/SCD and HOS/AB (P = 0.000). WBC in semen and Caspase 3 activity were negatively correlated with the percentage of normal sperm by using the HOS/EY test. Abnormal sperm with HOS/AB and HOS/SCD showed a negatively significant correlation with normal sperm by HOS/EY test, and WBC in semen showed a positively significant correlation with Caspase-3 activity (Table 4). The percentage of abnormal sperm by using the test of HOS/SCD showed a positively significant correlation with that of HOS/AB (r = 0.935, P = 0.000).
Table 4.
Correlation among sperm parameters of the conventional semen analysis, sperm DNA fragmentation, and abnormal sperm chromatin condensation, and normal sperm membrane and vitality, and Caspase 3 activity (n = 125)
| Variables | Sperm concentration | Sperm motility | Normal morphology | Abnormal HOS/SCD | Abnormal HOS/AB HOS/EY | Normal | Caspase-3 activity |
|---|---|---|---|---|---|---|---|
| Sperm concentration | r = 1 | r = 0.477a | r = 0.485a | r = −0.432a | r = −0.432a | r = 0.496a | r = −0.432a |
| Motility | r = 0.477a | r = 1 | r = 0.693a | r = −0.642a | r = −0.619a | r = 0.708a | r = −0.307a |
| Normal morphology | r = 0.485a | r = 0.693a | r = 1 | r = −0.775a | r = −0.772a | r = 0.810a | r = −0.424a |
| Abnormal HOS/SCD | r = −0.432a | r = −642a | r = −0.775a | r = 1 | r = 0.935a | r = −0.859a | r = 0.480a |
| Abnormal HOS/AB | r = −0.440a | r = −619a | r = −0.772a | r = 0.935a | r = 1 | r = −0.885a | r = 0.506a |
| Normal HOS/EY | r = 0.496a | r = 0.708a | r = 0.810 | r = −0.859a | r = −0.885a | r = 1 | r = −0.516a |
| Caspase3 activity | r = −0.320a | r = −0.307a | r = −0.424a | r = 0.480a | r = 0.506a | r = −0.516a | r = 1 |
aCorrelation is significant, P = 0.000 (2-tailed)
Discussion
Scrotal short-term heating, usually by immersing in a water bath is one of three local heating ways of the testes [28]. The most precise way of exposing a testis to an increased temperature for a short time is to immerse the scrotum in a water bath at the chosen temperature. This technique has been used in rats and monkeys. A negative correlation between scrotal temperature and spermatogenesis has been demonstrated. Local testicular heat treatment with 43 °C water induced reversible oligospermia or azoospermia in rodents and monkeys with increased germ cell apoptosis [28–34], and when the heat treatment stopped after 30 days, the sperm quantity could recover to the original level. The heat treatment of the scrotum was mainly through the impact on the metabolism and apoptosis of spermatogenic cells to decline the fertility of many kinds of animal models; moreover, this impact was temporary and reversible [30–33]. Previous experimental data from animal models suggested that scrotal heat stress may be a reversible method for contraception [35]. In boars, the number of spermatozoa was not affected in many heat experiments, but motility and the percentage of normal spermatozoa fell between 1 and 6 weeks after the heat exposure; when the heating was for only a limited period, motility and the percentage of normal spermatozoa recovered after about 6 weeks [36–40].
We designed the SHS bag with electric warming and which was attached to the underpants for men. The temperature was 40–43 °C and heating time was 40 min. After SHS 1 month, the parameters of sperm concentration, motility (grade “a” + “b” + “c”), normal morphology were significantly decreased compared with that before SHS, and the WBC in semen was significantly increased. The semen volume significantly decreased in samples from the group of SHS treated 3 months. After 3 months of recovery, the semen parameters (except sperm motility) gradually returned to prior level before SHS treatment. The sperm motility did not recover and the semen volume decreased 3 months after SHS treatment, which may due to the small size of our sample (19 subjects only). Larger sample is needed in the future study.
The testicular hyperthermia could cause a rapid and transient suppression of spermatogenesis and sperm DNA damage. Chihara et al. [41] reported heat-induced testicular damage although the MRL/MpJ-derived locus on Chr 1 may play a pivotal role in recovery from heat-induced testicular damage, especially via inhibition of calcification, MRL/MpJ mice have a precipitating factor for testicular calcification and heat shock-resistant factors that reside outside the 81-cM region of Chr 1. A study by [1, 42] revealed that hyperthermia resulted in a number of detrimental effects on the testis, including DNA damage in germ cells and mature sperm and a lengthy recovery period of the testis from mild transient heat shock. Other studies [31, 32] indicated that transient mild testicular hyperthermia results in azoospermia and oligozoospermia in monkeys through increased germ cell apoptosis with minimal effect on the hormonal milieu. Several studies in human populations have found a higher scrotal temperature in infertile men compared with fertile controls, and variation in scrotal temperature may contribute to the well known variation in semen quality [43]. In man, raised scrotal temperature can result in a negative correlation between high scrotal temperature and sperm output with sperm concentration being decreased 40 % per 1 °C increment of median day time scrotal temperature in a study of 99 men [43]. The heat stress not only directly induced the apoptosis of spermatogenic cells, but also impacted the cell division of the Sertoli cells to promote the apoptosis of spermatogenic cells [11, 44–46]. Studies indicated heat stress caused apoptosis in a large number of germ cells and reduced testicular confrontation demand for compensatory ability. In many cell types, hypoxia and oxidative stress have been shown to trigger apoptosis and cell death. One of the hallmarks of apoptosis is the fragmentation of sperm DNA [31, 32]. In present study, when the SHS treatment was carried out 1, 2 and 3 months, the rate of the hypotonic swelling and unstained spermatozoa (the rate of HOS/EY) were significantly declined, and the rate of the DNA-damaged sperm and abnormal chromatin condensation sperm as well as Caspase-3 activity also markedly increased than that before SHS (P < 0.001). Three months after SHS treatment, the above-mentioned indicators were restored to the level before the SHS experiment. It prompts that the spermatogenesis might be a transient damage, when the SHS experiment stopped for a period of time, the spermatogenesis will recover. Wang et al. reported that after heat stress treatment, Caspase-3, −8, −9 enzyme activities in newt testis were significantly elevated after heatshock (40 °C 2 h) [47]. In non-apoptotic cells, caspase activated deoxyribonuclease (CAD) is present as an inactive complex with iCAD. During apoptosis, caspase-3 cleaves the inhibitor, allowing the nuclease to cut the chromatin. In mouse testes following a single mild transient scrotal heat exposure (40 °C or 42 °C for 30 min), an increase in expression of the effector caspase cleaved Caspase-3 and a decrease in expression of the protein inhibitor of caspase-activated DNase (ICAD) were caused to germ cell death. Reduced expression of ICAD contributes to increased activity of caspase-activated DNase and is consistent with the increased rates of DNA fragmentation [1]. When 8-week-old mice exposed to a single scrotal heat treatment (42 °C for 25 min), the testes displayed severe damage, with multinucleated giant cells, nuclear condensation and germ cell loss in the seminiferous epithelium, and the number of cleaved Caspase-3positive germ cells per tubule was dramatically increased [1, 42].
In the present study, we observed changes in sperm parameters, sperm DNA fragmentation and sperm chromatin condensation and Caspase-3 of the programme of the SHS. When the programme of the SHS performed for 1, 2 and 3 months, the sperm concentration, sperm motility rate and morphologically normal sperm rate were significant decreased and WBC in semen was increased than that of pre-SHS (according to WHO 2010 standard [25], sperm concentration less than 15 × 106/ml in 31.6 %, 36.8 % and 26.3 %; sperm motility rate less than 40 % in 68.4 %, 79.0 % and 68.4 %; and morphologically normal sperm rate less than 4 % in 26.3 %, 52.6% and 57.9 %; respectively). The alteration of sperm concentration was lower than that sperm motility rate and morphologically normal sperm rate. The degree of changes in the sperm concentration, sperm motility rate and morphologically normal sperm rate after starting SHS for 1 and 2 months is greater than starting SHS for 3 months. It suggests that the testes may be gradually adapted to the local environment of the scrotal hyperthermia. After stooped SHS 3 months, the sperm concentration and morphologically normal sperm rate gradually returned to normal levels, and the sperm motility rate did not completely returned. This may take a long time further observation. Sperm membrane integrity and vitality were used by HOS/EY. We observed that sperm concentration, motility and normal morphology were negatively correlated with the percentage of abnormal sperm by using the test of HOS/SCD and HOS/AB. WBC in semen and Caspase 3 activity were negatively correlated with the percentage of normal sperm by using the HOS/EY test. Abnormal sperm with HOS/AB and HOS/SCD showed a negatively significant correlation with normal sperm by HOS/EY test, and WBC showed a positively significant correlation with Caspase-3 activity. The result of HOS/SCD test showed a strong positively significant correlation with that of HOS/AB test (r = 0.935, P = 0.000). The normal spermatozoa in HOS/EY test, HOS/SCD test and HOS/AB test were membrane integrity and vitality, DNA integrity and chromatin not damaged. If the test only performed by one of the tests such as HOS, EY, SCD or AB, some damaged spermatozoa may be as the normal sperm. The AB staining specifies sperm residual histones and indicates anomalies in sperm chromatin condensation. In earlier studies an association was reported between sperm developmental arrest, as demonstrated by aniline blue staining of persistent histones, and the number of chromosomal aberrations in semen samples [48–51]. These data lead us to a more detailed study of the relationship between sperm nuclear maturity and sperm membrane integrity and vitality as we pursued aniline blue staining and HOS within the same spermatozoon.
Acknowledgments
The authors would like to thank doctor Feng Chen (Shandong Provincial Xintai Family Planning Service Station) and Hua-Qiang Liu (Shandong Provincial Pingyin Family Planning Service Station) for their help in semen processing and their technical assistance.
Footnotes
The work was supported by the National “China’s 12th 5-Year Plan (2011–2015)” of Science and Technology (No: 2012BAI31B08)
Capsule The continuously constant SHS can impact the semen quality, sperm DNA integrity, chromatin condensation and Caspase-3, and the combination of HOS plus AB test may simultaneously determine the integrity of membrane and chromatin condensation at the same spermatozoon.
References
- 1.Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction. 2008;136:73–84. doi: 10.1530/REP-08-0036. [DOI] [PubMed] [Google Scholar]
- 2.Banks S, King SA, Irvine DS, Saunders PT. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction. 2005;129:505–514. doi: 10.1530/rep.1.00531. [DOI] [PubMed] [Google Scholar]
- 3.Liu YX. Control of spermatogenesis in primate and prospect of male contraception. Arch Androl. 2005;51:77–92. doi: 10.1080/01485010490485768. [DOI] [PubMed] [Google Scholar]
- 4.Garolla A, Torino M, Sartini B, Cosci I, Patassini C, Carraro U, et al. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum Reprod. 2013;28:877–885. doi: 10.1093/humrep/det020. [DOI] [PubMed] [Google Scholar]
- 5.Shrivastava V, Pekar M, Grosser E, Im J, Vigodner M. SUMO proteins are involved in the stress response during spermatogenesis and are localized to DNA double-strand breaks in germ cells. Reproduction. 2010;139:999–1010. doi: 10.1530/REP-09-0492. [DOI] [PubMed] [Google Scholar]
- 6.Li XX, Chen SR, Shen B, Yang JL, Ji SY, Wen Q, et al. The Heat-Induced Reversible Change in the blood-testis barrier (BTB) is regulated by the androgen receptor (AR) via the partitioning-defective protein (Par) polarity complex in the mouse. Biol Reprod. 2013;89:1–10. doi: 10.1095/biolreprod.113.110478. [DOI] [PubMed] [Google Scholar]
- 7.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas mediated pathway. Exp Cell Res. 1999;251:350–355. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 8.Shen HM, Dai J, Chia SE, Lim A, Ong CN. Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum Reprod. 2002;17:1266–1273. doi: 10.1093/humrep/17.5.1266. [DOI] [PubMed] [Google Scholar]
- 9.Love CC, Kenney RM. Scrotal heat stress induces altered sperm chromatin structure associated with a decrease in protamine disulfide bonding in the stallion. Biol Reprod. 1999;60(3):615–620. doi: 10.1095/biolreprod60.3.615. [DOI] [PubMed] [Google Scholar]
- 10.Karabinus DS, Vogler CJ, Saacke RG, Evenson DP. Chromatin structural changes in sperm after scrotal insulation of Holstein bulls. J Androl. 1997;18(5):549–555. [PubMed] [Google Scholar]
- 11.Wang C, Cui YG, Wang XH, Jia Y, et al. Transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab. 2007;92(8):3292–3304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y, Wang LG, Jia YF, Yang DT, Zhang MH, Zhang YP, et al. The effects of the extract of Chinese Polygala tennuidolia willd on human sperm in vitro. J Zhejiang Univ Sci B. 2011;12:448–454. doi: 10.1631/jzus.B1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Y, Wang LG, Zhang LH, Li J, Zhang AD, Zhang MH. Sperm chromosomal aneuploidy and DNA integrity of infertile men with anejaculation. J Assist Reprod Genet. 2012;29:185–194. doi: 10.1007/s10815-011-9688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Y, Wang LG, Zhang LH, Zhang AD. Quality of sperm obtained by penile vibratory bratory stimulation and percutaneous vasal sperm aspiration in men with spinal cord injury. J Androl. 2012;33:1036–1046. doi: 10.2164/jandrol.111.014902. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LH, Qiu Y, Wang KH, Wang Q, Tao G, Wang LG. Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nick-end labeling assay. Fertil Steril. 2010;94:102732. doi: 10.1016/j.fertnstert.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Fariello RM, Del Giudice PT, Spaine DM, Fraietta R, Bertolla RP, Cedenho AP. Effect of leukocytospermia and processing by discontinuous density gradient on sperm nuclear DNA fragmentation and mitochondrial activity. J Assist Reprod Genet. 2009;26:151–157. doi: 10.1007/s10815-008-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu DY, Baker HW. Human sperm bound to the zona pellucida have normal nuclear chromatin as assessed by acridine orange fluorescence. Hum Reprod. 2007;22:1597–1602. doi: 10.1093/humrep/dem044. [DOI] [PubMed] [Google Scholar]
- 18.Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 19.Fernández JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–842. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 20.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980;210:1131. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 21.Paasch U, Grunewald S, Fitzl G, Glander HJ. Deterioration of plasma membrane is associated with activation of caspases in human spermatozoa. J Androl. 2003;24:246–252. doi: 10.1002/j.1939-4640.2003.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 22.Faleiro L, Lazebnik Y. Caspases disrupt the nuclear-cytoplasmic barrier. J Cell Biol. 2000;151:951–959. doi: 10.1083/jcb.151.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyret E, Lin H. Pinpointing the expression of piRNAs and function of the PIWI protein subfamily during spermatogenesis in the mouse. Dev Biol. 2011;14215–26. [DOI] [PMC free article] [PubMed]
- 24.World Health Organisation . Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. New York: Cambridge University Press; 1999. [Google Scholar]
- 25.World Health Organisation 2010 WHO laboratory manual for the Examination and processing of human semen. 5th ed. WHO Press, Prepublication version. p30–32.
- 26.Sellami A, Chakroun N, Ben Zarrouk S, Sellami H, Kebaili S, Rebai T, et al. Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Adv Urol. 2013;578–631. [DOI] [PMC free article] [PubMed]
- 27.Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30:1519–1524. doi: 10.1007/s10815-013-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setchell BP. The Parkes Lecture. Heat and the testis. J Reprod Fertil. 1998;114:179–194. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- 29.Liu YX. Temperature control of spermatogenesis and prospect of male contraception. Front Biosci (Schol Ed) 2010;1:730–755. doi: 10.2741/s97. [DOI] [PubMed] [Google Scholar]
- 30.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 31.Lue Y, Hikim AP, Wang C, Im M, Leung A, Swerdloff RS. Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the “two-hit” approach to male contraceptive development. Endocrinology. 2000;141:1414–1424. doi: 10.1210/endo.141.4.7416. [DOI] [PubMed] [Google Scholar]
- 32.Lue Y, Wang C, Liu Y-X, Hikim AP, Zhang XS, Ng CM, et al. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91:539–545. doi: 10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- 33.Guo J, Tao SX, Chen M, Shi YQ, Zhang ZQ, Li YC, et al. Heat treatment induces liver receptor homolog-1 expression in monkey and rat Sertoli cells. Endocrinology. 2007;131:1137–1148. doi: 10.1210/en.2006-1004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XS, Lue YH, Guo SH, Yuan JX, Hu ZY, Han CS, et al. Expression of HSP105 and HSP60 during germ cell apoptosis in the heat-treated testes of adult cynomolgus monkeys (MACACA FASCICULARIS) Front Biosci. 2005;10:3110–3121. doi: 10.2741/1767. [DOI] [PubMed] [Google Scholar]
- 35.Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988;49:1–23. doi: 10.1016/s0015-0282(16)59640-x. [DOI] [PubMed] [Google Scholar]
- 36.McNitt JL, First NL. Effects of 72-hour heat stress on semen quality in boars. Int J Biometeorol. 1970;14:373–380. doi: 10.1007/BF01462914. [DOI] [PubMed] [Google Scholar]
- 37.Wettemann RP, Wells ME, Johnson RK. Reproductive characteristics of boars during and after exposure to increased ambient temperature. J Anim Sci. 1979;49:1501–1505. [Google Scholar]
- 38.Stone BA. Thermal characteristics of the testis and epididymis of the boar. J Reprod Fertil. 1981;63:551–557. doi: 10.1530/jrf.0.0630551. [DOI] [PubMed] [Google Scholar]
- 39.Larsson K, Einarsson S. Seminal changes in boars after heat stress. Acta Vet Scand. 1984;25:57–66. doi: 10.1186/BF03547279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malmgren L, Larsson K. Semen quality and fertility after heat stress in boars. Acta Vet Scand. 1984;25:425–435. doi: 10.1186/BF03547257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chihara M, Nakamura T, Sakakibara N, Otsuka S, Ichii O, Kon Y. The onset of heat-induced testicular calcification in mice: involvement of the telomeric locus on chromosome 1. Am J Pathol. 2014;184:2480–2492. doi: 10.1016/j.ajpath.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–919. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hjollund NH, Storgaard L, Ernst E, Bonde JP, Olsen J. Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol. 2002;16:215–221. doi: 10.1016/S0890-6238(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZH, Hu ZY, Song XX, Xiao LJ, Zou RJ, Han CS, et al. Disrupted expression of intermediate filaments in the testis of rhesus monkey after experimental cryptorchidism. Int J Androl. 2004;27:234–239. doi: 10.1111/j.1365-2605.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Cai H, Yang JL, Lu CL, Liu T, Yang W, et al. Effect of heat stress on expression of junction-associated molecules and upstream factors androgen receptor and Wilms’ tumor 1 in monkey sertoli cells. Endocrinology. 2008;149:4871–4882. doi: 10.1210/en.2007-1093. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Yuan JX, Shi YQ, Zhang XS, Hu ZY, Gao F, et al. Effect of 43 degrees treatment on expression of heat shock proteins 105, 70 and 60 in cultured monkey Sertoli cells. Asian J Androl. 2008;10:474–485. doi: 10.1111/j.1745-7262.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang DH, Hu JR, Wang LY, Hu YJ, Tan FQ, Zhou H, et al. The apoptotic function analysis of p53, Apaf1, Caspase3 and Caspase7 during the spermatogenesis of the Chinese fire-bellied newt Cynops orientalis. PLoS One. 2012;7e39920. [DOI] [PMC free article] [PubMed]
- 48.Simon L, Liu L, Murphy K, Ge S, Hotaling J, Aston KI, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod. 2014;29:904–917. doi: 10.1093/humrep/deu040. [DOI] [PubMed] [Google Scholar]
- 49.Dadoune JP. The nuclear status of human sperm cells. Micron. 1995;26:323–345. doi: 10.1016/0968-4328(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 50.Morel F, Mercier S, Roux C, Elmrini T, Clavequin MC, Bresson JL. Interindividual variations in the disomy frequencies of human spermatozoa and their correlation with nuclear maturity as evaluated by aniline blue staining. Fertil Steril. 1998;69:1122–1127. doi: 10.1016/S0015-0282(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 51.Morel F, Roux C, Bresson JL. Disomy frequency estimated by multicolour fluorescence in situ hybridization, degree of nuclear maturity and teratozoospermia in human spermatozoa. Reproduction. 2001;121:783–789. doi: 10.1530/rep.0.1210783. [DOI] [PubMed] [Google Scholar]