Figure 6.
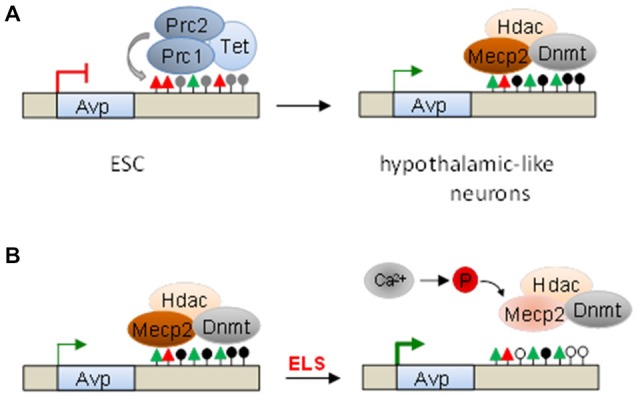
Serial epigenetic switches underlie early-life stress (ELS)-dependent programming of Avp. (A) Polycomb complexes (Prc1 and Prc2) occupy the downstream Avp enhancer in undifferentiated embryonic stem cells (ESC). The histone methyltransferase Suz12, the catalytic subunit of Prc2, and ten-eleven translocation (Tet) proteins catalyze H3K27me3 (red triangles) and 5hmC (gray lollipops), respectively. Upon hypothalamic-like differentiation, PcG and Tet proteins dissociate, while de novo DNA methyltransferases (Dnmt) enter and promote Avp enhancer methylation. This allows binding of the methyl-CpG-binding protein 2 (Mecp2) and associated repressor complexes, which modulate Avp expression. (B) ELS-driven neuronal activity triggers calcium-calmodulin kinase-dependent Mecp2-S421 phosphorylation, enhancer dissociation, and Avp derepression. Loss of Mecp2 and associated Dnmts tilts the balance between methylation and postnatal methylome reconfiguration towards demethylation thus leaving an enduring memory trace of the initial event.
