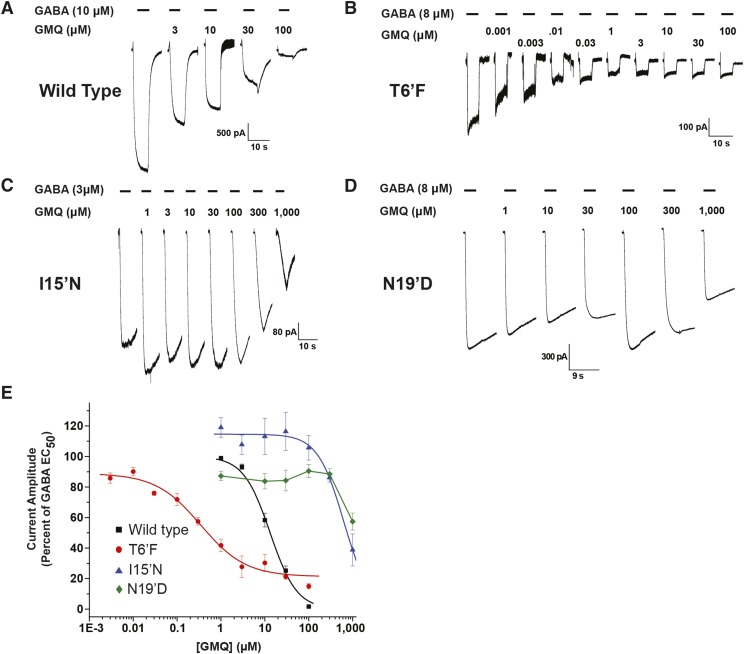
Fig. 4.
Human GABA-A ρ1 receptor containing the 15′ and 6′ mutations display altered response profiles to GMQ. (A) Representative traces of hGABA-A ρ1 receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (B) Representative traces of hGABA-A ρ1 T6′F mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (C) Representative traces hGABA-A ρ1 I15′N mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. All activation currents generated by applying GABA and GMQ test solutions for 5 seconds. (D) Representative traces of hGABA-A ρ1 N19′D mutant receptor currents elicited upon coapplication of GABA EC50 with increasing concentrations of GMQ. (E) Comparison of normalized concentration-response profiles of GMQ inhibition in wild-type hGABA-A ρ1 and hGABA-A ρ1 I15′N and hGABA-A T6′F mutant receptors compared with the respective control concentration of GABA. The determined GMQ IC50 was 13.2 ± 0.6 μM with a Hill coefficient of 1.4 ± 0.1 for the wild-type hGABA-A ρ1 receptor, 0.4 ± 0.1 μM with a Hill coefficient of 0.8 ± 0.2 for the hGABA-A ρ1 T6′F mutant receptor, and 630.5 ± 58.4 μM with a Hill coefficient of 1.5 ± 0.2 for the hGABA-A ρ1 I15′N mutant receptor. We were unable to fit the data for the N19′D mutation, and thus no IC50 was obtained. Data are presented as the mean ± S.E.M., with a sample size of n ≥ 5 cells.