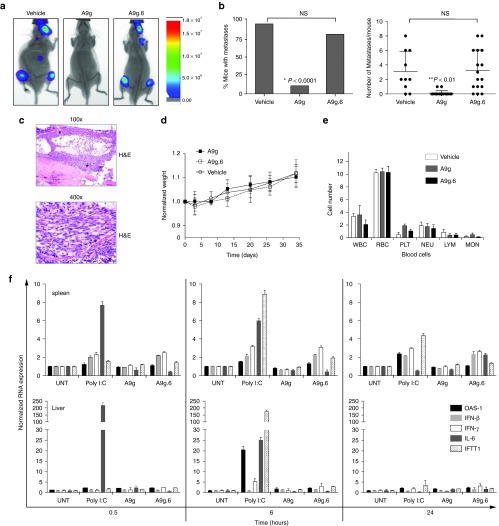
Figure 3.
In vivo efficacy and safety of A9g PSMA aptamer. (a) Representative images of vehicle (n = 10), A9g (n = 18) and A9g.6 (n = 16) treated SCID mice following intra-cardiac injection of luciferase (Luc+) expressing PSMA+ prostate cancer cells, 22Rv1(1.7). Images were acquired on the AMI 1000 Instrument (Spectral Instruments Imaging). (b) (Left panel) Percentage of mice with metastases from the three treatment groups (vehicle, A9g or A9g.6) 4 weeks after intra-cardiac injection of 22rv1(1.7) cells (*P < 0.0001, Fisher's exact test comparing A9g-treated group to either vehicle or A9g.6-treated groups). (Right panel) Number of bioluminescent (Luc+) foci (metastatic foci) per mouse, per group was quantified. (**P < 0.001, Student t-test comparing A9g-treated group to either vehicle or A9g.6-treated groups) (c) Representative histological section of bone with disseminated disease. Hematoxylin and Eosin (H&E) staining of bone section at 100× and 400× shown * = indicates bone metastases. (d) Effect of A9g aptamer on mouse weight during course of treatment. Normalized weight: weights normalized to pretreatment values. (e) Effect of A9g on blood cells after a complete treatment course. CBC is reported for white blood cells (WBC), red blood cells (RBC), platelets (PLT), neutrophils (NEU), lymphocytes (LYM), and monocytes (MON). (f) Assessment of potential immune stimulatory effect of A9g in immune-competent mice. Poly I:C: positive control for immune stimulation. Spleens (top panels) and livers (bottom panels) of treated mice were collected at the indicated time points and processed for total RNA. Expression levels of several immune responsive genes were determined by RT-qPCR. OAS-1: 2′-5′ oligoadenylate synthetase 1A, IFN-β: interferon beta 1, IFN-γ: interferon gamma, IL-6: interleukin 6, and IFIT1: interferon-induced protein with tetratricopeptide repeats 1.