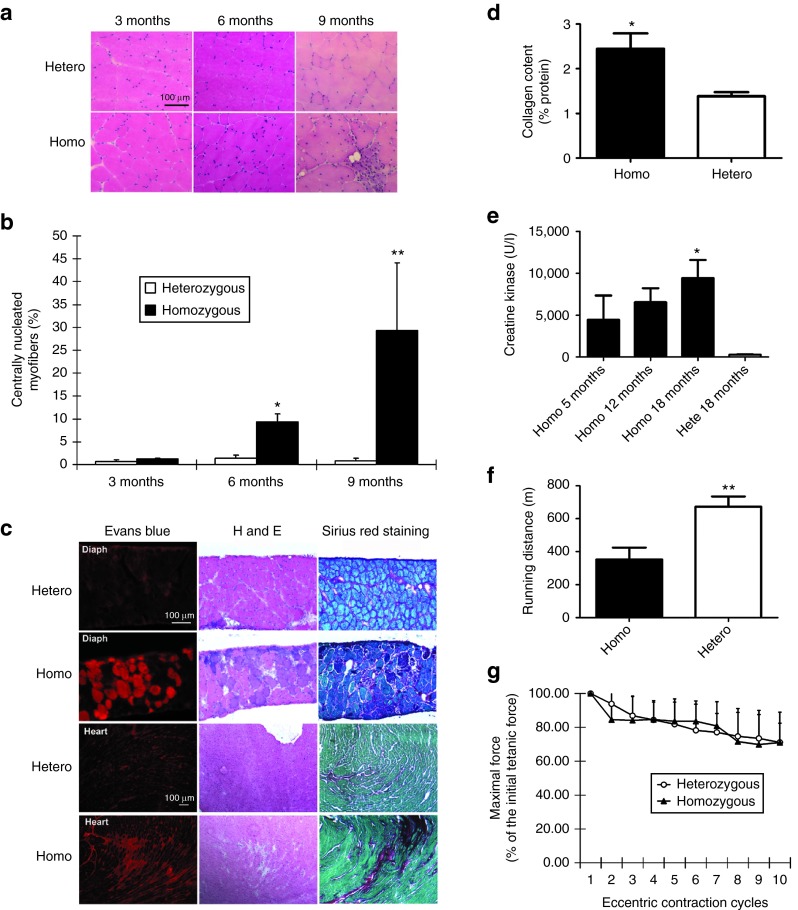
Figure 1.
Characterization of homozygous FKRP L276IKImice. (a) Muscle pathology corresponding to age was displayed for both heterozygous and B6 L276IKI homozygous mice. Cryo-thin-sections of the quadriceps muscle from different ages were analyzed with H&E staining. Large amount of centrally nucleated myofibers were observed in the quadriceps of both the 6- and 9-month-old B6 L276IKI homozygous mice compared to the heterozygous mice. (b) Quantitation of centrally nucleated myofibers in homozygous versus heterozygous mice at different ages (n = 8; *P < 0.05; **P < 0.001). (c) Muscle leakage and fibrosis progression seen in 18 months old homozygous FKRP L276I mice. The diaphragm of homozygous FKRP L276IKI mice (18-month-old) displayed the most dramatic dystrophic pathology as revealed by the Evans blue dye leakage, mononuclear cell infiltration (H&E staining), and fibrosis infiltration (collagen staining). Sirius red was used to stain collagen (red), and Fast green was utilized for myofiber staining (green). (d) Quantification of collagen content in the diaphragm muscle shown in Figure 1c. The collagen content was expressed as a percentage of the collagen (Sirius red staining) versus the noncollagen protein (fast green staining). Statistics were calculated by an unpaired t-test with Welch's correction (*P < 0.05; n = 4). (e) The serum creatine kinase level was increased in homozygous mice. All homozygous B6 L276IKI mice had CK levels greater than 1,000 units/l, and revealed a trend that the serum CK levels increase with age (*P < 0.05 with one-way analysis of variance (ANOVA); n = 4–9). (f) The treadmill running experiment indicated homozygous mice ran shorter distance than heterozygous control (**P = 0.01 with one-way ANOVA; n = 5–9). (g) The in vitro eccentric contraction force measurement. There was no difference in force production after 10 cycles of eccentric contraction challenge in the TA muscles of the homozygous mice versus heterozygous mice (n = 6, P > 0.05).