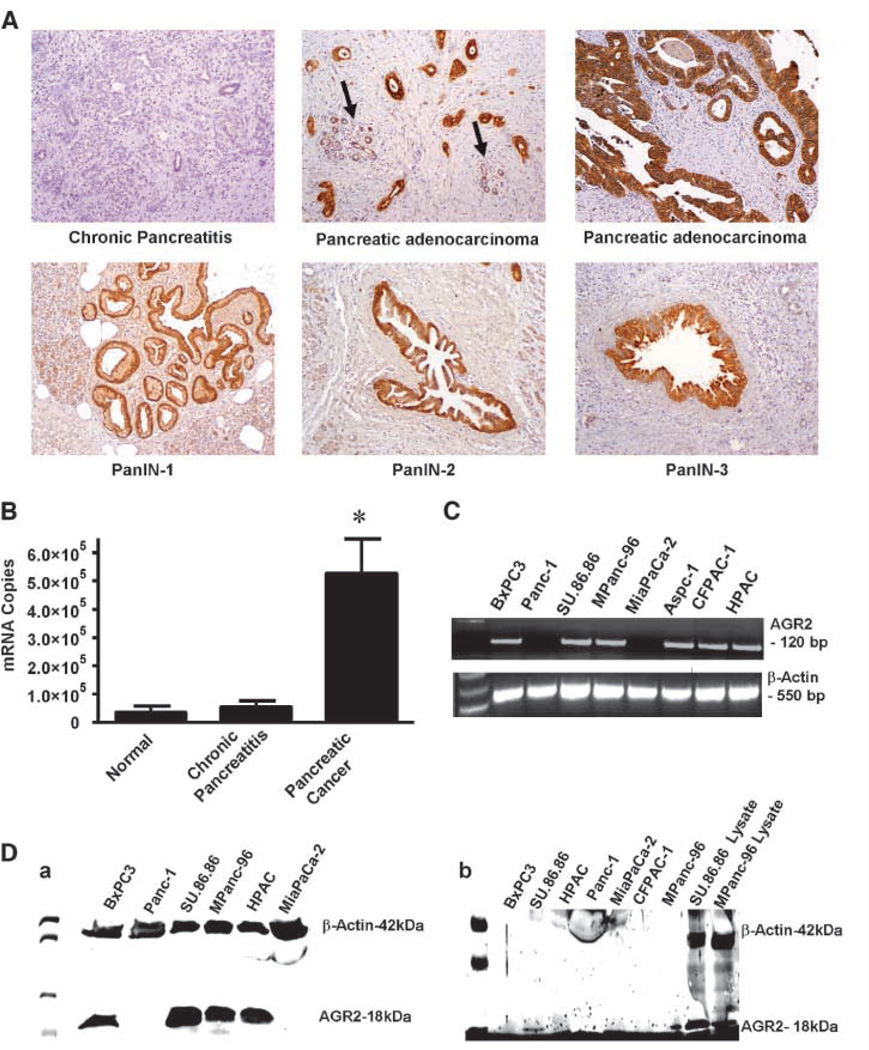
Figure 1.
AGR2 is expressed in pancreatic cancer tissues and cell lines and secreted. A, AGR2 was analyzed in samples of chronic pancreatitis, pancreatic adenocarcinoma, and PanIN lesions. Chronic pancreatitis samples did not stain for AGR2. In contrast, pancreatic adenocarcinomas showed strong positive staining for AGR2 (the arrow indicates low level of AGR2 expression in adjacent benign pancreatic tissue). High levels of AGR2 expression were also found in PanIN-1, PanIN-2, and PanIN-3. B, Q-RT-PCR results are shown for AGR2 mRNA in normal, chronic pancreatitis and pancreatic adenocarcinoma tissues. C, RT-PCR showing the expression of AGR2 mRNA in human pancreatic cancer cell lines. AGR2 mRNA was present in all cell lines except Panc-1 and MiaPaCa-2 (lanes 2 and 5). Water served as blank, and β-actin served as loading control. A full-length gel is presented in Supplementary Fig. S1. D, a, Western blot showing the presence of AGR2 protein in cell lysates of all human pancreatic cancer cell lines, except Panc-1 and MiaPaCa-2 (lanes 2 and 6). AGR2 was not present in normal pancreas tissue. β-Actin was used as a loading control. AGR2 was identified as an 18-kDa protein. A full-length gel is presented in Supplementary Fig. S2. b, Western blot showing the presence of AGR2 protein in conditional media and lysates of human pancreatic cancer cell lines. Significant levels of AGR2 were observed in the conditioned media from SU.86.86 and MPanc-96 (lanes 1 and 7, respectively) but not other cell lines. Lysates from SU.86.86 and MPanc-96 served as positive controls (lanes 8 and 9, respectively). β-Actin was detected in the lysates but not in the conditioned media that was positive for AGR2. Micrograph shown is representative of three independent experiments. A full-length gel is presented in Supplementary Fig. S3.