Figure 1.
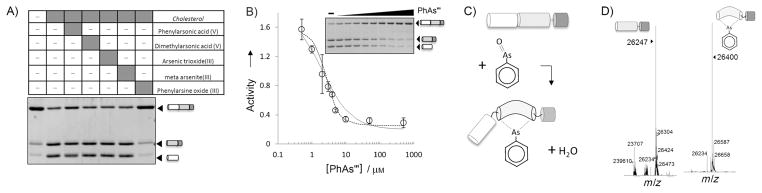
Identification of PhAsIII as a tight-binding inhibitor of Hh cholesterolysis. A) Screen of arsenical compounds as potential inhibitors of Hh cholesterolysis activity. Hh precursor (4 μM) was preincubated with indicated arsenical compounds (60 μm, final) before initiating reaction with cholesterol (500 μM). Samples were electrophoresed on 15% SDS-polyacrylamide gels, stained with Coomassie Brilliant Blue, and digitized with a BioRad imager. B) Titration of Hh activity by PhAsIII. Activity of Hh precursor (2 μM) was determined by the extent of processing as in (A). Data were fit to a standard dose–response curve (—) by using an IC50 value of 2 μM, as well as to a quadratic binding equation (····) by using a Ki value of 0.4 μM. Error bars indicate the data range (n=2). C) Proposed mechanism for covalent interation of PhAsIII and Hh precursor. D) Detection of Hh–PhAsIII adduct by mass spectrometry. Deconvoluted mass of autocatalytic HhC (10 μM) without (left) and with (right) 1 equiv of Hh–PhAsIII. Additional mass is consistent with a condensation mechanism (expected mass change: 154; observed mass change: 153).
