Figure 2.
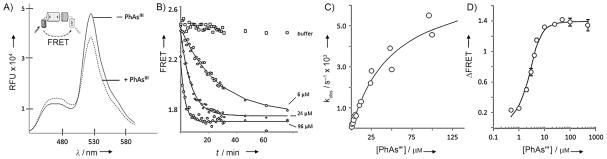
Real-time detection of PhAsIII binding by Hh using an optical reporter. A) Effect of PhAsIII on the fluorescence of FRET-active Hh precursor protein. Spectra of precursor, C-H-Y, in aqueous buffered solution (pH 7.1) with and without 1 equiv of added PhAsIII (λex=400 nm, λem=420–600 nm). Inset: Schematic of FRET-active construct with cyan fluorescent protein fused to the N terminus of the HhC domain and yellow fluorescent protein fused to the C terminus. B) Kinetics of PhAsIII binding by C-H-Y measured by FRET change. Time traces showing loss of FRET signal from C-H-Y after addition of the indicated concentration of PhAsIII. Solid lines show first-order exponential decay (kobs), calculated by using best-fit values. C) Bimolecular kinetics of PhAsIII binding by C-H-Y is saturable. Rate constants (kobs [s−1]) for FRET loss plotted as a function of increasing PhAsIII concentration. Solid line shows the expected behavior, assuming reversible formation of a noncovalent encounter complex, followed by irreversible formation of the PhAsIII/C-H-Y complex. D) Fluorometric titration of binding of PhAsIII to C-H-Y. Plot shows the overall FRET change (FRET○–FRETf) as a function of PhAsIII concentration. Solid line shows the expected behavior for an inhibitor with a EC50 value of 0.4 μM. Error bars indicate data range (n=3)
