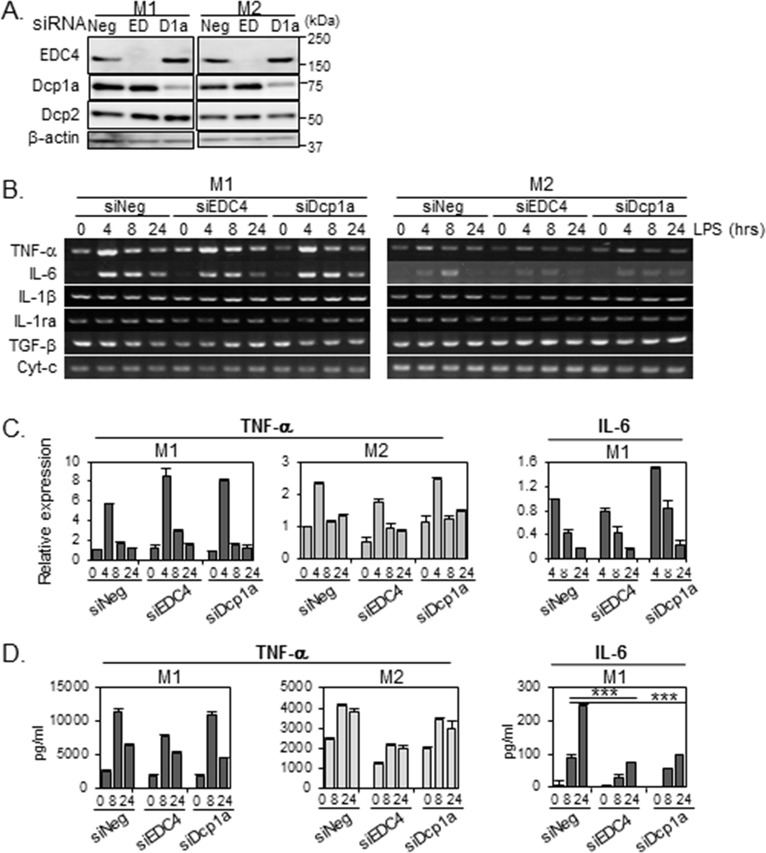
Fig 6. P-body knockdown reduces LPS-stimulated IL-6 release in M1-THPs.
THP-1 cells were transfected with siRNA targeting EDC4 (EDC), Dcp1a (D1a), or with a non-targeting siRNA (siNeg). Twenty hours after transfection, the cells were PMA-differentiated, activated toward an M1 or M2 phenotype, and subjected to LPS stimulation as described in Methods. (A) Depletion of EDC4 or Dcp1a by siRNA. The siRNA-transfected cells were polarized toward an M1 or M2 phenotype, harvested, and subjected to immunoblot analysis with the indicated antibodies; β-actin served as a loading control. (B) Effects of EDC4 or Dcp1a knockdown on cytokine mRNA expression. The siRNA-transfected cells were polarized toward an M1 or M2 phenotype, treated with LPS, harvested at the time points indicated, and analyzed by semi-quantitative RT-PCR. (C) P-body knockdown did not affect the levels of LPS-induced IL-6 and TNF-α mRNA expression in M1-THPs. Total RNA was isolated 0, 4, 8, or 24 hours after LPS stimulation and subjected to quantitative real-time RT-PCR analysis to detect IL-6 and TNF-α gene transcripts, as described in Methods. RNAs were normalized to HPRT RNA. The level of the IL-6 and TNF-α transcripts in siNeg-transfected cells at 4 and at 0 hours after LPS stimulation, respectively, was set to 1 to calculate the relative values in each sample. The IL-6 mRNA level before LPS stimulation (0 hour) was below the limit of detection. Data are averages of three independent experiments; error bars indicate SD. (D) P-body knockdown reduced the level of IL-6 released from M1-THPs cells. Supernatants were harvested at 0, 8, or 24 hours after LPS stimulation, and protein concentrations of IL-6 and TNF-α were measured by ELISA. IL-6 protein levels before LPS stimulation (0 hour) were below the limit of detection. Data are averages of three independent experiments; error bars indicate SD. ***p<0.001