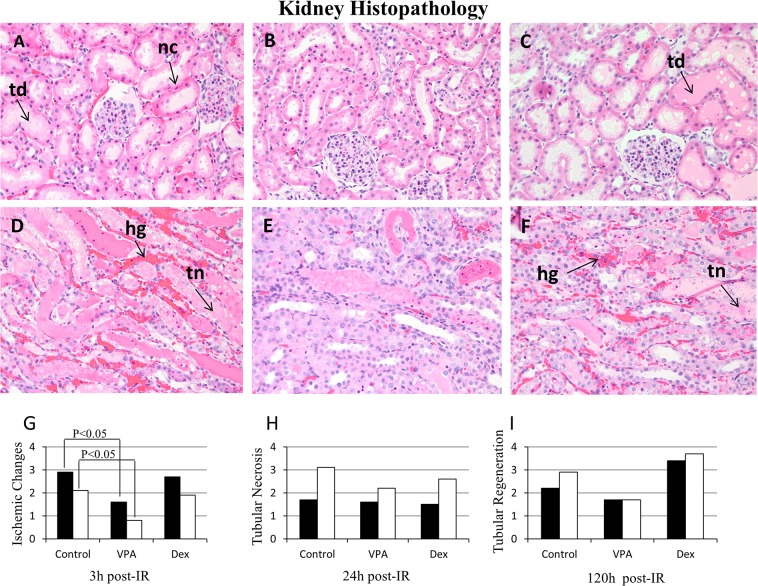
Fig 3. Histopathology of hematoxylin and eosin stained kidney sections.
A, B, C = Renal cortex at 3 hours (h) post ischemia-reperfusion (IR); D, E, F = Renal outer medulla at 24 h post-IR; A, D = Vehicle (saline control); B, E = Valproic acid (VPA); C, F = Dexamethasone (Dex) treated animals. Three high power fields (400x) representing approximately 50 tubules from cortex and outer medulla of each kidney were evaluated for ischemic changes (injury), tubular necrosis and regenerative changes. Collectively kidney injury and regeneration were graded (0–4) based on the mean percentage of tubules affected: 0, None; 1, <25%; 2, ≥25 but <50%; 3, ≥50 but <75%; 4, >75–100%. Ischemic changes included nuclear condensation (nc), cytoplasmic eosinophilia, individual cell necrosis and tubular dilation (td); tubular necrosis (tn) included confluent cell necrosis or sloughing of the tubular epithelium; and regenerative changes included tubular dilation, cytoplasmic basophilia and contraction of the cytoplasm, as well as vesicular chromatin with nucleoli. Hemorrhage (hg) was predominant in the vehicle control group. G, H, I = represent Histopathology quantification: renal cortex (black bars ■) and renal outer medulla (white bars □). The histologic injury score was significantly (P<0.05) lower in the VPA treated group compared to the Vehicle control at 3 h post-IR (see Table 3).