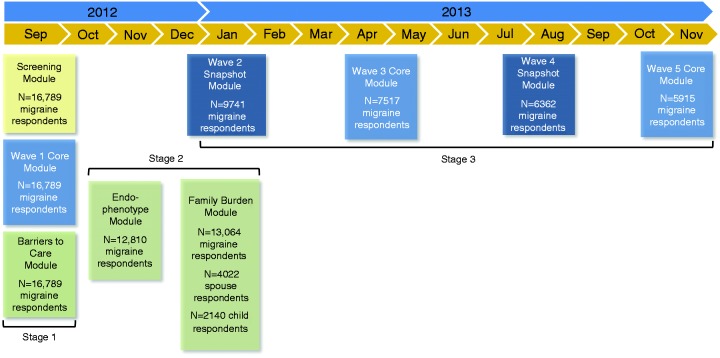
Figure 2.
Study design.
N = number of returns for that module only, and does not represent a running total of participation in previous modules.
Module completion dates are as follows. Stage 1: Screening, Wave 1 Core, and Barriers to Care, September 17–October 30, 2012. Stage 2: Comorbidities/Endophenotypes, October 10–December 17, 2012; at the conclusion of wave 4 and before start of wave 5, nonrespondents to the Comorbidities/Endophenotypes Module were resampled (August 19–October 3, 2013). Family Burden-Proband, November 14, 2012–January 28, 2013. Family Burden-Partner, November 30, 2012–October 30, 2013. Family Burden-Child, January 11–October 30, 2013; at conclusion of wave 3 and before wave 4, nonrespondents to the Family Burden Survey were resampled (Proband, April 22–September 4, 2013; Partner, May 7–October 30, 2013; Child: May 3–October 30, 2013). Stage 3: Wave 2 Snapshot, December 21, 2012–February 19, 2013. Wave 3 Core, March 20–May 15, 2013. Wave 4 Snapshot, June 20–August 19, 2013. Wave 5 Core, September 19–November 19, 2013.
For Wave 1 Core, no cases could attrit; the total returns screening positive for migraine were n = 17,000 out of n = 58,629 (these cases plus the n = 10,044 who were over-quota and n = 12,110 who abandoned produce the total returns of n = 80,783); of these, n = 211 (1.2%) were removed during cleaning, resulting in a final sample of n = 16,789 qualified respondents. All five waves of longitudinal assessments were completed by n = 3626 of n = 16,789 (21.6%) respondents; four of five waves by n = 2364 (14.1%), three of five waves by n = 2415 (14.4%), two of five waves by n = 3109 (18.5%), and only one wave (i.e. baseline) by n = 5275 (31.4%).