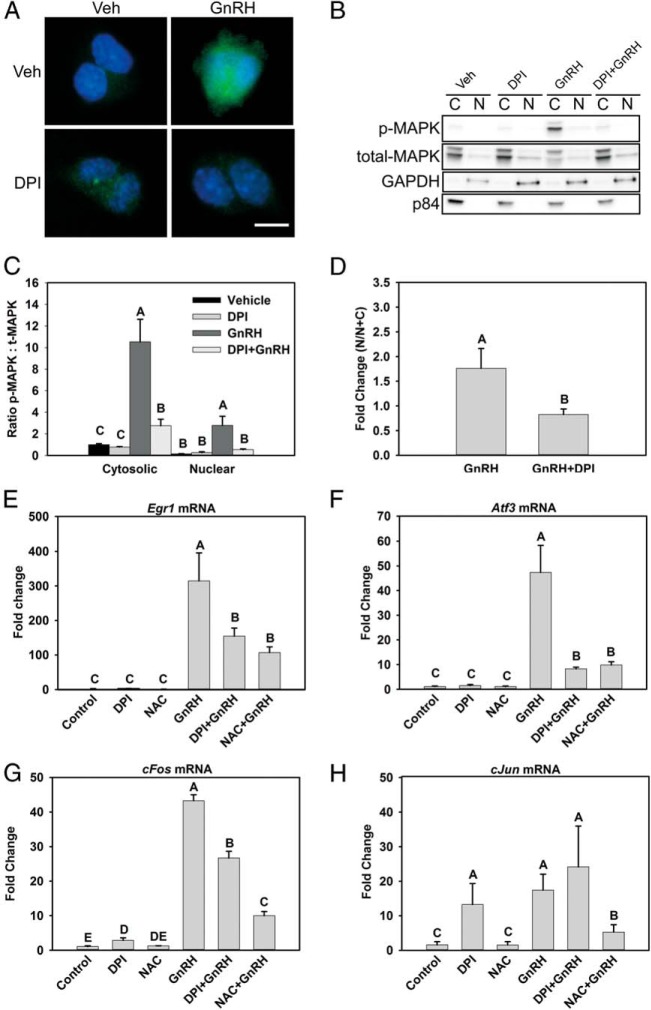
Figure 5.
NOX/DUOX inhibition suppresses nuclear MAPK1/3 and IEG induction by GnRH. A, MAPK1/3 activation in both nuclear and cytosolic compartments was examined by immunocytochemical analysis of LβT2 cells treated with 10 nM GnRH for 30 minutes with or without preincubation with the NOX/DUOX inhibitor DPI. Cells were fixed and stained with an antibody to phosphorylated MAPK1/3 and Alexa Fluor 488–conjugated secondary antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole-containing mounting medium before visualization (blue) B, LβT2 cells treated similarly were harvested and fractionated into nuclear (N) and cytosolic (C) fractions and evaluated by Western blotting for abundance of phosphorylated (p) MAPK1/3. C, Blots were stripped and reblotted for total MAPK1/3 and quantified by chemiluminescence. Blots of subcellular fractions were also probed with antibodies for GAPDH and p84 to distinguish separation of cytosolic and nuclear fractions, respectively. D, Nuclear localization of phosphorylated MAPK1/3 appeared disproportionately reduced by DPI, so the fold increase in nuclear phospho-MAPK1/3 was specifically evaluated in vehicle and DPI-treated samples. To analyze IEG mRNA responses, serum-starved LβT2 cells were preincubated with 10 mM NAC or 5 μM DPI for 30 minutes. After treatment, the cells were incubated with 10 nM GnRH for 1 hour. Real-time PCR was performed using specific primers for Egr1 (E), Atf3 (F), c-Fos (G), and c-Jun (H). GAPDH mRNA was used to normalize samples, and each gene is shown relative to the vehicle control. Data are reported as means ± SEM from 3 independent experiments. Letters represent significant differences (P < .05) by ANOVA followed by the Tukey multiple comparison test. Groups with different letters are significantly different from each other.