Fig. 1.
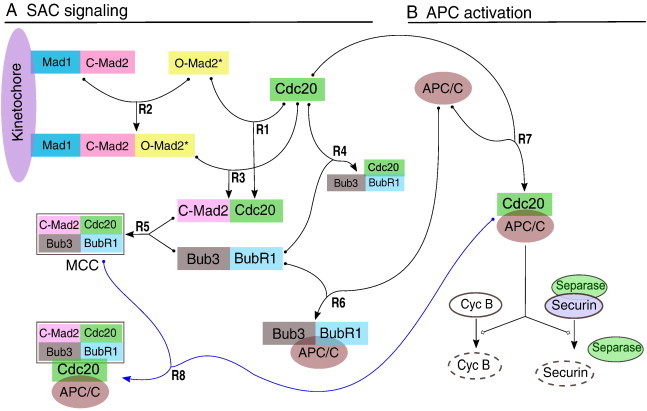
Schematic representation of the core mechanism of SAC.
(A) The SAC acts mainly through sequestration of the APC/C-activator Cdc20 by Mad2. Mad2 in closed conformation (C-Mad2) anchored at the kinetochore via Mad1 recruits cytosolic Mad2 in open conformation (O-Mad2). The so recruited Mad2 is stabilized in an intermediate conformation (Mad2*), which in turn is able to bind Cdc20 efficiently. The resulting C-Mad2–Cdc20 dimers are released from the kinetochore and form the mitotic checkpoint complex (MCC) together with Bub3 and BubR1. The Cdc20-containing complexes are not stable and dissociate with a certain rate, thus Cdc20 becomes available for APC/C activation soon after the last signaling kinetochore is silenced by proper microtubule attachment. (B) When SAC signaling is turned off, Cdc20 binds to and thereby activates the APC/C. Active APC/C:Cdc20 promotes degradation of securin, which leads to cohesin cleavage by now active separase. The resulting separation of sister-chromatids is the hallmark of anaphase. Simultaneously, APC/C:Cdc20 promotes degradation of cyclin B, a requirement for mitotic exit.
