Figure 5.
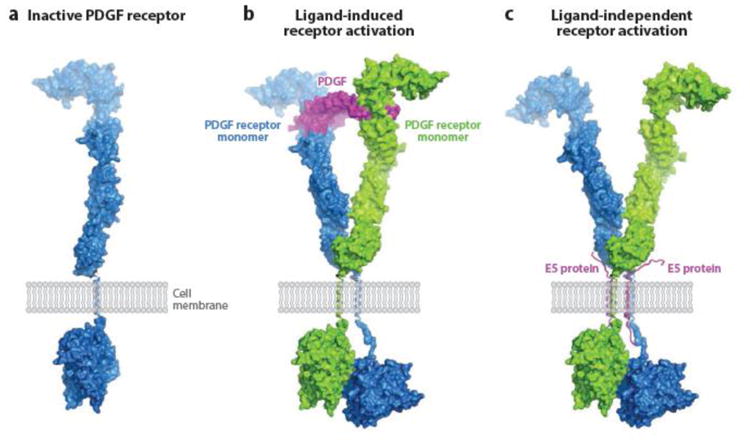
Model for E5-induced PDGF receptor activation. (a) Surface representation of monomeric inactive PDGF β receptor, showing the extracellular ligand-binding domain, the single transmembrane domain (shown as a single helical ribbon), and the intracellular kinase domain in an inactive, closed conformation. (b) PDGF receptor activation induced by PDGF binding. Pre formed PDGF dimer (center) binds to the extracellular domains of two PDGF receptor monomers and thereby facilitates dimerization via tight contacts, which allow one of the intracellular kinase domains to maintain an active, open conformation, ready to catalyze transphosphorylation. (c) PDGF receptor activation induced by BPV E5 binding. Instead of imposing PDGF receptor dimerization through ligand binding as seen in (b), BPV E5 (two transmembrane helixes) promotes receptor dimerization through direct contacts with the receptor transmembrane segments. The following structures were used to generate these models: crystal structure of PDGF-BB in complex with the ligand-binding domains of PDGF β receptor (108a), crystal structures of the ectodomain of c-Kit before and after SCF binding (140c), and 3-D cryoEM reconstruction of the entire c-Kit transmembrane receptor in complex with SCF (82a). Abbreviations: PDGF, platelet-derived growth factor; SCF, stem cell factor. Figure prepared by Yarden Opatowsky (Bar-Ilan University) and adapted from Reference 108 (copyright 2010 National Academy of Sciences, USA) and Reference 140c (copyright 2007 Elsevier), with permission.
