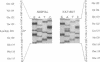Abstract
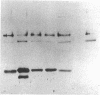
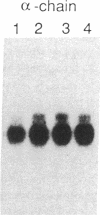
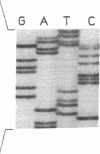
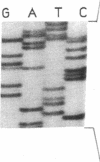
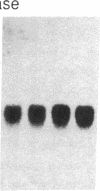
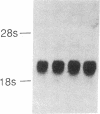
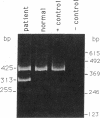
Sandhoff disease is caused by mutations affecting the beta subunit of lysosomal beta-hexosaminidase (EC 3.2.1.52) and displays a wide spectrum of clinical phenotypes. We report a 57-year-old patient with a very mild phenotype, although residual hexosaminidase A activity in his cultured fibroblasts was less than 3% of normal activity, a level observed in juvenile onset patients. Northern and Western blot analyses confirmed a similar low level of beta subunit-mRNA and mature beta-protein, respectively. Two mutations of the HEXB gene were identified in this patient, a partial 5' gene deletion (a null allele), and a C----T transition 8 nucleotides downstream from the intron 10/exon 11 junction affecting the splicing of the beta subunit-mRNA. In their homozygous forms, the 5' deletion has been previously shown to result in a severe infantile phenotype, and the C----T transition in a juvenile phenotype. The genotype and the low level of residual hexosaminidase A activity would be expected to produce a juvenile Sandhoff phenotype in this patient, as well as in four of his six clinically normal siblings. The biochemical basis of his mild phenotype is uncertain, but may result from genetic variations in the RNA splicing machinery.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayleran J., Hechtman P., Saray W. Synthesis of 4-methylumbelliferyl-beta-D-N-acetylglucosamine-6-sulfate and its use in classification of GM2 gangliosidosis genotypes. Clin Chim Acta. 1984 Nov 15;143(2):73–89. doi: 10.1016/0009-8981(84)90215-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983;6(1):58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- Guise K. S., Korneluk R. G., Waye J., Lamhonwah A. M., Quan F., Palmer R., Ganschow R. E., Sly W. S., Gravel R. A. Isolation and expression in Escherichia coli of a cDNA clone encoding human beta-glucuronidase. Gene. 1985;34(1):105–110. doi: 10.1016/0378-1119(85)90300-2. [DOI] [PubMed] [Google Scholar]
- Hasilik A., von Figura K., Conzelmann E., Nehrkorn H., Sandhoff K. Lysosomal enzyme precursors in human fibroblasts. Activation of cathepsin D precursor in vitro and activity of beta-hexosaminidase A precursor towards ganglioside GM2. Eur J Biochem. 1982 Jul;125(2):317–321. doi: 10.1111/j.1432-1033.1982.tb06685.x. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., O'Brien J. S. Separation of glycoprotein-derived oligosaccharides by thin-layer chromatography. Anal Biochem. 1979 Feb;93(1):167–170. [PubMed] [Google Scholar]
- Hubbes M., Callahan J., Gravel R., Mahuran D. The amino-terminal sequences in the pro-alpha and -beta polypeptides of human lysosomal beta-hexosaminidase A and B are retained in the mature isozymes. FEBS Lett. 1989 Jun 5;249(2):316–320. doi: 10.1016/0014-5793(89)80649-0. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Kaback M. M., Nathan T. J., Greenwald S. Tay-Sachs disease: heterozygote screening and prenatal diagnosis--U.S. experience and world perspective. Prog Clin Biol Res. 1977;18:13–36. [PubMed] [Google Scholar]
- Kytzia H. J., Sandhoff K. Evidence for two different active sites on human beta-hexosaminidase A. Interaction of GM2 activator protein with beta-hexosaminidase A. J Biol Chem. 1985 Jun 25;260(12):7568–7572. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowden J. A., Skomorowski M. A., Henderson F., Kaback M. Automated assay of hexosaminidases in serum. Clin Chem. 1973 Dec;19(12):1345–1349. [PubMed] [Google Scholar]
- Mahuran D. J., Neote K., Klavins M. H., Leung A., Gravel R. A. Proteolytic processing of pro-alpha and pro-beta precursors from human beta-hexosaminidase. Generation of the mature alpha and beta a beta b subunits. J Biol Chem. 1988 Apr 5;263(10):4612–4618. [PubMed] [Google Scholar]
- Mahuran D. J. The biochemistry of HEXA and HEXB gene mutations causing GM2 gangliosidosis. Biochim Biophys Acta. 1991 Feb 22;1096(2):87–94. doi: 10.1016/0925-4439(91)90044-a. [DOI] [PubMed] [Google Scholar]
- Nakano T., Suzuki K. Genetic cause of a juvenile form of Sandhoff disease. Abnormal splicing of beta-hexosaminidase beta chain gene transcript due to a point mutation within intron 12. J Biol Chem. 1989 Mar 25;264(9):5155–5158. [PubMed] [Google Scholar]
- Neote K., Bapat B., Dumbrille-Ross A., Troxel C., Schuster S. M., Mahuran D. J., Gravel R. A. Characterization of the human HEXB gene encoding lysosomal beta-hexosaminidase. Genomics. 1988 Nov;3(4):279–286. doi: 10.1016/0888-7543(88)90116-4. [DOI] [PubMed] [Google Scholar]
- Neote K., McInnes B., Mahuran D. J., Gravel R. A. Structure and distribution of an Alu-type deletion mutation in Sandhoff disease. J Clin Invest. 1990 Nov;86(5):1524–1531. doi: 10.1172/JCI114871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Cumming D. A., Gravel R. A., Mahuran D. Oligosaccharide structure and amino acid sequence of the major glycopeptides of mature human beta-hexosaminidase. Biochemistry. 1988 Jul 12;27(14):5216–5226. doi: 10.1021/bi00414a041. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Rubin M., Karpati G., Wolfe L. S., Carpenter S., Klavins M. H., Mahuran D. J. Adult onset motor neuronopathy in the juvenile type of hexosaminidase A and B deficiency. J Neurol Sci. 1988 Oct;87(1):103–119. doi: 10.1016/0022-510x(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Takeshita K., Forget B. G., Scarpa A., Benz E. J., Jr Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984 Jul;64(1):13–22. [PubMed] [Google Scholar]
- Wakamatsu N., Kobayashi H., Miyatake T., Tsuji S. A novel exon mutation in the human beta-hexosaminidase beta subunit gene affects 3' splice site selection. J Biol Chem. 1992 Feb 5;267(4):2406–2413. [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., MacDougall B. G. Juvenile Sandhoff disease: some properties of the residual hexosaminidase in cultured fibroblasts. Am J Hum Genet. 1976 Sep;28(5):489–495. [PMC free article] [PubMed] [Google Scholar]