Figure 1.
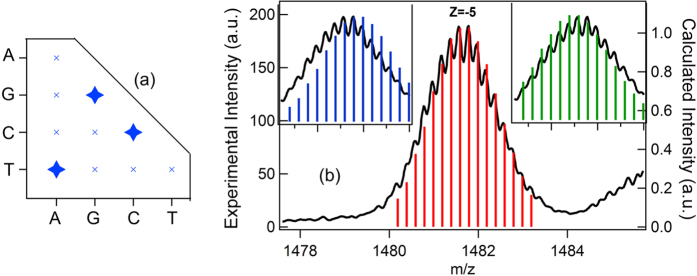
(a) Schematic of the homo-base strand types and combinations studied. Stars denote the detected Ag+-bridged duplexes. (b) Example of isotope peak envelope resolved in MS for C11-(Ag+)11-C11. Black lines: data. The total mass of the ionized species (m) is given by m = mDNA + mAgNAg – npr, where mDNA is the mass of the unionized DNA strand, mAgNAg is the mass of the total silver content, and npr is the number of protons removed by negative mode electrospray ionization. The charge state, z (negative) of the ionized species is z = QAg/e – npr, where QAg is the total charge associated with the silver content. Bars show the calculated isotope peak patterns for a net charge on the silver content of QAg = +10e (blue), +12e (green)(insets) and +11e (red) associated with the silver atom content. The best fit at a charge of +11e confirms that all of the attached silver atoms are cations, Ag+.
