Summary
Impulsive and aggressive behaviors are both modulated by serotonergic signaling, specifically through the serotonin 1B receptor (5-HT1BR). 5-HT1BR knockout mice show increased aggression and impulsivity, and 5-HT1BR polymorphisms are associated with aggression and drug addiction in humans. To dissect the mechanisms by which the 5-HT1BR affects these phenotypes, we developed a mouse model to spatially and temporally regulate 5-HT1BR expression. Our results demonstrate that forebrain 5-HT1B heteroreceptors expressed during an early postnatal period contribute to the development of the neural systems underlying adult aggression. However, distinct heteroreceptors acting during adulthood are involved in mediating impulsivity. Correlating with the impulsivity, dopamine in the nucleus accumbens is elevated in the absence of 5-HT1BRs, and normalized following adult rescue of the receptor. Overall, these data show that while adolescent expression of 5-HT1BRs influences aggressive behavior, a distinct set of 5-HT1B receptors modulate impulsive behavior during adulthood.
Introduction
Impulsivity and aggression are related behavioral phenotypes that are both modulated by serotonin (Coccaro, 1992). Although highly comorbid, frequently affected by the same manipulations, and often referred to as a single trait– impulsive-aggression (Balleine and Dickinson, 1998; Dalley et al., 2002)– they are distinct behavioral constructs with potentially discrete underlying neural systems (Garcia-Forero et al., 2009). Little is known about how serotonin modulates the underlying neural circuits, or whether there is a developmental contribution to these effects. Studies in humans and a variety of animal models suggest that increased serotonergic signaling during development is associated with increased adult aggressive and impulsive behavior (Cases et al., 1995; Dennis et al., 2013; Ricci and Melloni, 2012). However, the reverse is true in adulthood– serotonin levels are inversely correlated with aggression, impulsivity and risky behavior (Audero et al., 2013; Brown et al., 1979; Crockett et al., 2009; Higley and Linnoila, 1997). These differential effects may be mediated either by distinct underlying neural circuits and/or different sensitive periods. Since aggression and impulsivity are intermediate phenotypes in a number of mental health disorders, including addictive disorders, antisocial personality disorder, and attention deficit disorder, understanding the underlying neural circuit(s) may help develop more effective treatments.
The serotonin 1B receptor (5-HT1BR) is implicated in both aggressive and impulsive behavior. In humans, polymorphisms in 5-HT1BR have been linked to impulsive aggression, anger, conduct disorder, and other disorders which have dysregulated impulse control such as substance use disorders (Cao et al., 2013; Conner et al., 2010; Jensen et al., 2009; Zouk et al., 2007). In mouse models, 5-HT1BR knockout (KO) mice are highly aggressive and impulsive. Specifically, conspecific male territorial aggression is increased– KO residents attack intruders more often and with a shorter latency (Saudou et al., 1994). KO mice also exhibit increased cocaine self-administration and low response inhibition (Pattij et al., 2003; Rocha et al., 1998). While these studies implicate 5-HT1BR in modulating adult aggressive and impulsive behavior, they do not address the potential developmental impact of 5-HT1BR, nor delineate the underlying neural circuits.
The 5-HT1BR is a G protein coupled receptor (GPCR) that inhibits neurotransmitter release from both serotonergic and non-serotonergic neurons (reviewed in Sari, 2004). The ‘autoreceptor’ population of 5-HT1BRs is located on the axon terminals of serotonergic cells, thus affecting the release of serotonin in many brain regions. The ‘heteroreceptor’ population of 5-HT1BRs is located on nerve terminals of neurons from other neurotransmitter systems including glutamate, GABA, dopamine, and acetylcholine (Boschert et al., 1994; Pazos et al., 1985). In all cases, the receptor is synthesized in the cell body and transported to pre-synaptic terminals, where it is coupled to either the G protein Gi or Go (Boschert et al., 1994), and inhibits neurotransmitter release (Mizutani et al., 2006).
To dissect the role of the 5-HT1BR in mediating aggressive and impulsive behavior, we have generated a targeted transgenic mouse that permits temporal and spatial regulation of 5-HT1BR (Figure 1). A floxed tetracycline operator (tetO)-5-HT1B cDNA cassette has been inserted in place of the endogenous coding region of htr1b to generate Htr1btetO/tetO mice (referred to as tetO1B). Crossing tetO1B mice to transgenic mice expressing either the tetracycline-dependent transcriptional silencer (tTS) or Cre recombinase allows knock-down of htr1b expression. Here we have used the ubiquitous β-actin promoter and two tissue specific promoters– CaMKIIα and Pet1– to drive 5-HT1BR knockdown of all receptors, forebrain heteroreceptors, or autoreceptors, respectively. Our data show that ubiquitous knockdown results in aggressive and impulsive behavior (in keeping with the original knockout phenotype). However, rescue of receptor expression in adulthood with doxycycline reverses the impulsive, but not the aggressive, phenotype. This suggests that adult aggression can be determined early during development, while impulsivity can be modulated throughout adulthood. Furthermore, CaMKIIα mediated forebrain knockdown results in aggressive, but not impulsive, behavior. Overall, our data indicate that the 5-HT1BR mediates aggressive and impulsive behavior through modulation of distinct circuits at different times.
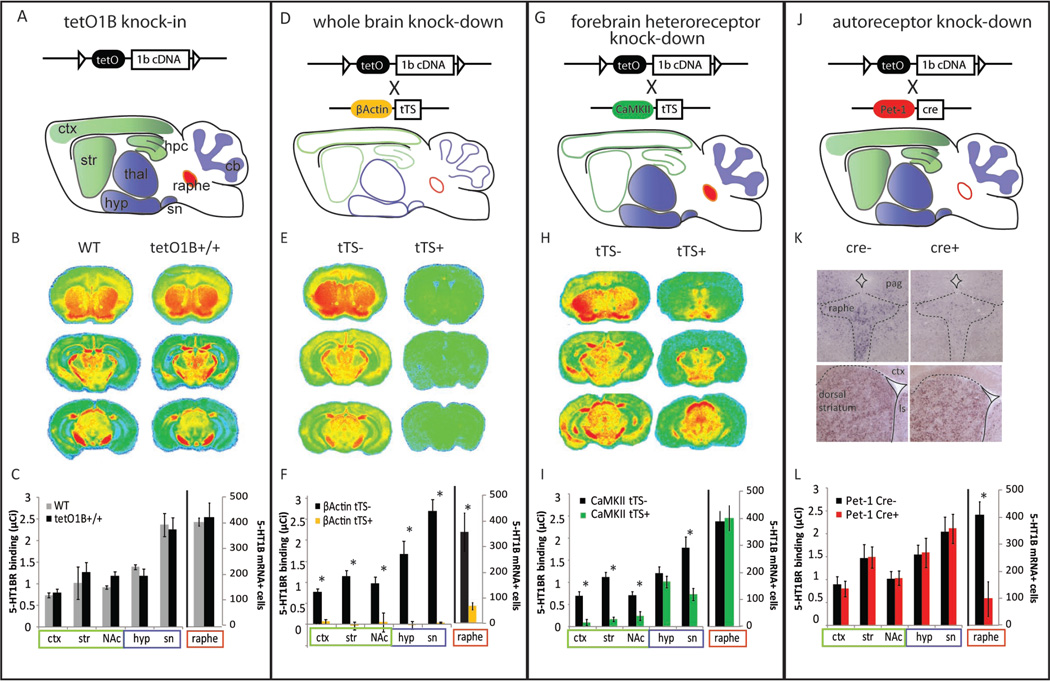
Figure 1. Characterization of the tissue-specific regulation of 5-HT1BR expression in the floxed tetO1B transgenic mouse model.
Top) Schematics of transgenic constructs used to manipulate 5-HT1B receptor expression are shown for single tetO1B+/+ transgenic (A), β-actin-tTS+::tetO1B+/+ (D), CaMKII-tTS+::tetO1B+/+ (G), and ePet-Cre+::tetO1B+/+ (J) mice. Cartoons of brains illustrate regions of 5-HT1BR mRNA, including cortex (ctx), dorsal striatum (str), hippocampus (hpc), hypothalamus (hyp), thalamus (thal), substantia nigra (sn), raphe, and cerebellum (cb). Regions of mRNA knockdown are indicated for each manipulation by a lack of shading. Middle) Receptor autoradiographs show heat map distribution of 125I-cyanopindolol receptor binding in three representative coronal sections throughout the brain, rostral at bregma +0.5mm, mid at bregma −2.3mm, caudal at bregma −3.5mm for tetO1B+/+ (B), β-actin-tTS+::tetO1B+/+ (E), and CaMKII-tTS+::tetO1B+/+ (H) mice. Color scheme from red to blue indicates highest to lowest binding. Representative photomicrographs show DIG-labeled 5-HT1BR mRNA in the raphe, and the dorsal striatum as a control region, for ePet-Cre- and ePet-Cre+ mice (K).
Bottom) Quantification of receptor binding and 5-HT1B mRNA expression for tetO1B+/+ (C), β-actin-tTS+::tetO1B+/+ (F), CaMKII-tTS+::tetO1B+/+ (I), and ePet-Cre+::tetO1B+/+ (L) mice are represented as group means ± SEM. Left axis shows levels of receptor binding in cortex, dorsal striatum, nucleus accumbens, hypothalamus and substantia nigra. The number of 5-HT1BR mRNA+ cells in the raphe are shown for each transgenic mouse line and littermate controls on the right axis. *, p<0.05. See also Figure S1.
Results
Generation and characterization of tissue-specific and conditional 5-HT1BR transgenic mouse
Insertion of the floxed tetO1B cassette (tet operator and htr1b cDNA) in place of the endogenous htr1b gene did not alter baseline expression of 5-HT1BR in the brain (Figure 1A–C, and S1A–C, E; F1,6=0.01, p>0.05;). Crossing tetO1B mice to β-actin-tTS mice (forthwith referred to as β-actin-tTS mice; Mallo et al., 2003) yielded mice with ubiquitous knockdown of 5-HT1BR (Figure 1D). Throughout life, these mice had an absence of all receptor binding throughout the brain (Figure 1E–F, and S1D, F; tTS- vs tTS+: F1,6=58.92, p<0.001; tTS+ vs zero: t83=0.66, p>0.05). For tissue specific knockdown, tetO1B mice were crossed to CaMKIIα-tTS mice (Richardson-Jones et al., 2011) to assess the contribution of heteroreceptors. CaMKIIα-tTS+::tetO1B+/+ (forthwith referred to as CaMKII-tTS+) showed postnatal knockdown of the expression of one subset of forebrain receptors in the striatum, hippocampus, and cortex (Figure 1G). This resulted in a decrease of 5-HT1BR binding in striatum, hippocampus, cortex, substantia nigra, subthalamic nucleus, olfactory tubercle, and globus pallidus by PN 21 (Figure 1H–I, S1G–H and S3C; F1,6>10.90, p<0.05). 5-HT1BR binding was unaffected in other brain regions measured which represent the remaining population of 5-HT1B heteroreceptors (Figure S1G; F1,6=0.51, p>0.05). These results demonstrate the ability to spatially regulate 5-HT1BR expression in the tetO1B mice.
TetO1B mice were also crossed to mice expressing the ePet-Cre transgene (Scott et al., 2005; B6.Cg-Tg(Fev-cre)1Esd/J, stock#012712; The Jackson Laboratory, Bar Harbor, ME). ePet-Cre::tetO1B+/+ mice (forthwith referred to as ePet-Cre mice) had normal 5-HT1BR expression in all brain regions as measured by receptor autoradiography (F1,6=0.19, p>0.05); however, any differences in receptor binding in ePet-Cre+ mice were likely obscured due to the fact that autoreceptors are distributed widely at relatively low density throughout brain regions which also contain high expression of heteroreceptors (Figure 1J). To determine the extent of autoreceptor knockdown, 5-HT1BR mRNA was assessed in the raphe. The number of 5-HT1B mRNA positive cells was reduced in the raphe, but not other brain regions, of ePet-Cre+ mice (Figure 1K–L; F1,6=44.30, p<0.001). Because some mRNA expression remained in the raphe, we confirmed the functional effect of autoreceptor knockdown using serotonin microdialysis (Figure S1I; t20=4.69, p<0.001).
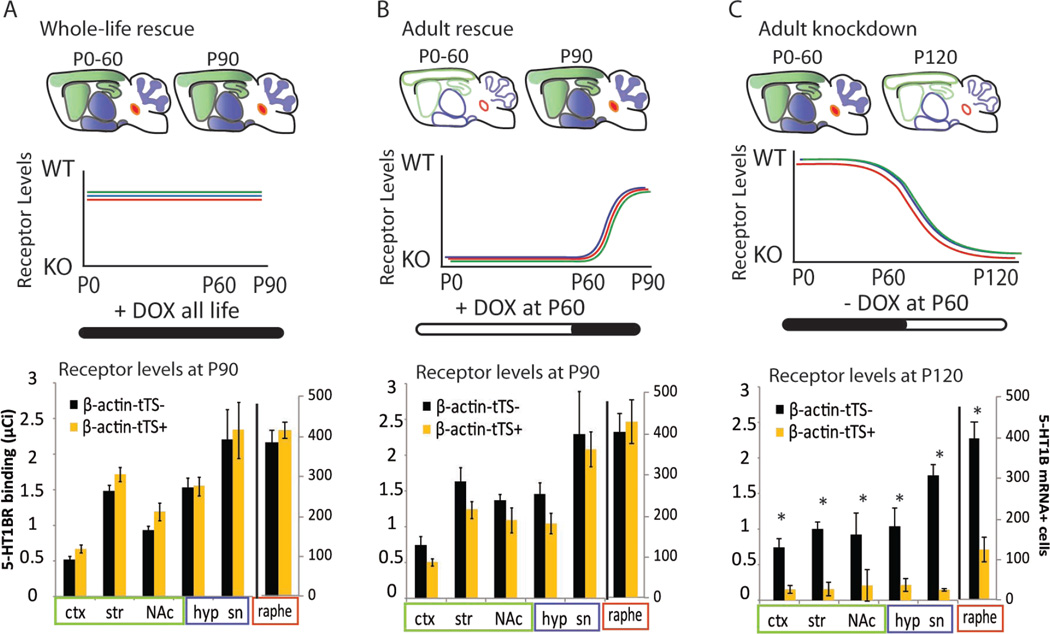
Receptor expression was also regulated temporally in β-actin-tTS mice by administering doxycycline in the food. Whole-life administration of doxycycline to β-actin-tTS+ mice resulted in rescue of the receptor throughout the brain (Figure 2A and S2A–B, G; β-actin-tTS+ with doxycycline vs tTS- with doxycycline: F1,6=0.10, p>0.05). Additionally, administration of doxycycline beginning in adulthood rescued receptor expression throughout the brain (Figure 2B and S2C–D, H; F1,9=0.61, p>0.05). A time course analysis of the rescue of receptor expression revealed that expression returned to normal levels by 21d after beginning doxycycline administration (Figure S3A). Adult knockdown was also possible in the β-actin line through withdrawal of doxycycline at P60. Although the knockdown was not as complete as whole-life knockdown (receptor binding values were significantly different from 0 at P120, t83=6.78, p<0.001), there was a significant reduction compared to littermate tTS- controls (Figure 2C and S2E–F, I; F1,6=68.63, p<0.001).
Figure 2. Characterization of the temporal regulation of 5-HT1BR expression in the floxed tetO1B transgenic mouse model.
Diagrams are shown for the approximate time course of receptor expression in the brain of β-actin-tTS+ mice following doxycycline treatment throughout life or beginning in adulthood. Schematic charts show protein levels of autoreceptors (red), heteroreceptors in CaMKII+ cells (green), and all other heteroreceptors (blue) throughout development. Bars below chart depict the timing of doxycycline administration, with black indicating the presence of doxycycline and white indicating its absence. The quantification of receptor binding (left) and the number of 5-HT1BR mRNA+ cells (right) are shown for β-actin-tTS+ mice and β-actin-tTS- controls following whole life rescue (A), adult rescue (B), and adult knockdown (C). Data is presented as group means ± SEM. See also Figure S2 and S3.
Aggressive behavior is mediated by developmental expression of forebrain 5-HT1B heteroreceptors
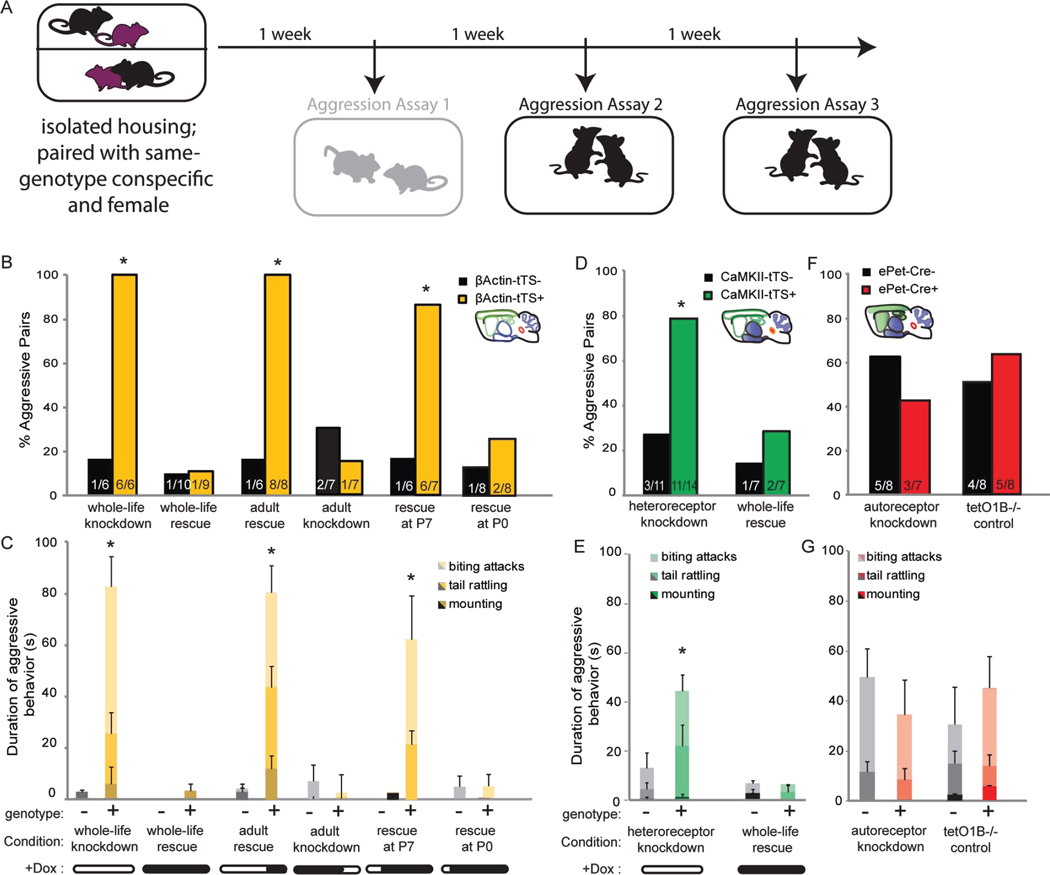
β-actin-tTS+ mice which lacked all 5-HT1BR throughout life were more aggressive compared to β-actin-tTS- tetO1B littermates (Figure 3A), which is consistent with the phenotype seen in constitutive 5-HT1B KO mice (Saudou et al., 1994). All pairs of β-actin-tTS+ mice (6 out of 6) displayed aggressive behavior, while only one pair of tTS- mice showed aggression (Figure 3B; χ21=8.57, p<0.01). β-actin-tTS+ duration of aggression was also increased– on average displaying aggressive behaviors including mounting, tail rattling, and/or biting attacks for over a minute out of the 5 minute session (Figure 3C; F1,10=25.13, p<0.05). Compared to controls, the number of attacks was also increased in β-actin-tTS+ mice (Figure S4A; F1,10=8.31, p<0.05). Their latency to attack was also significantly lower (Figure S4B; χ21=10.0, p<0.005), but there was no significant difference in the duration of non-aggressive social behavior compared to controls (Figure S4G; F1,10=2.04, p>0.05). Whole-life rescue of 5-HT1BR expression in β-actin-tTS+ mice (dox all life) rescued behavior to control levels of aggression. Specifically, only 1 out of 9 β-actin-tTS+ pairs on doxycycline showed aggressive behavior, which was not significantly different compared to their β-actin-tTS- littermates (Figure 3B; χ21=0.01, p>0.05).
Figure 3. Developmental expression of 5-HT1B heteroreceptors contributes to aggressive behavior in adult male mice.
A) Schematic of experimental paradigm used for isolation-induced aggression assay which includes co-housing with females for the first week, and three subsequent interactions. Data from first interaction (shaded grey) is not included in analysis to allow for stabilization of aggressive behavior. B) The percentage of pairs of mice showing aggressive behavior is shown for β-actin-tTS-control mice (black) and littermate β-actin-tTS+ mice (yellow) in six experimental conditions: no doxycycline all life (whole-life knockdown), all-life doxycycline treatment (whole-life rescue), doxycycline treatment beginning at P60 (adult rescue), doxycycline administrations ending at P60 (adult knockdown) and doxycycline treatment beginning at P7 or P0 (rescue at P7 or P0). C) Duration of aggressive behavior is shown, divided by type of behavior including biting, tail rattling, and mounting, for β-actin-tTS- control mice (shades of black) and β-actin-tTS+ (shades of yellow), presented as group means ± SEM. D, F) Percentage of pairs of CaMKII-tTS+ (green) or ePet-Cre+ (red) mice showing aggressive behavior compared to pairs of littermate controls (black). E, G) Duration of aggressive behavior is shown for CaMKII-tTS+ (shades of green), ePet-Cre+ (shades of red), and control mice (shades of black). *, p<0.05. See also Figure S4.
Interestingly, adult rescue of 5-HT1BR in β-actin-tTS+ mice did not rescue normal behavior. Following doxycycline treatment beginning at P60, all 8 of 8 β-actin-tTS+ pairs were aggressive, significantly more than β-actin-tTS- pairs (Figure 3B; χ21=10.37, p<0.01). β-actin-tTS+ pairs showed an average of over a minute of aggressive behavior during the 5 minute test, significantly more than β-actin-tTS- pairs (F1,12=16.76, p<0.05). This duration of aggression in β-actin-tTS+ mice treated in adulthood with doxycycline was comparable to that seen in β-actin-tTS+ mice in the absence of the receptor throughout life, suggesting no amelioration of the behavioral phenotype with adult rescue of the receptor. β-actin-tTS+ mice with adult rescue of the receptor also had an increased number of attacks and decreased latency to attack compared to β-actin-tTS- mice, but the duration of non-aggressive social behavior was unchanged (Figure S4G; F1,12=5.78, p<0.05; χ21=8.89, p<0.005; F1,12=0.19, p>0.05, respectively). Additionally, to ensure that aggression did not result from knocking down 5HT1BR for an extensive period of time (i.e. 60 days) regardless of the developmental stage, aggression was measured following adult knockdown of 5-HT1BR by removal of doxycycline at P60. Pairs of β-actin-tTS+ mice were not significantly more aggressive than tTS- control pairs (Figure 3B; χ21=0.42, p>0.05). These data suggest that developmental expression of 5-HT1BR is important for the regulation of adult aggressive behavior.
The inability to rescue the normal phenotype with adult rescue of the receptor was noteworthy, given pharmacology studies which show that activation of 5-HT1BRs with agonists (e.g. ‘serenics’) reduce adult aggressive behavior in mice (Fish et al., 1999). To address this, CP-94,253, a 5-HT1BR specific agonist, was injected intraperitoneally (i.p.) into β-actin-tTS+ mice with adult rescue of the receptor. CP-94,253 injection reduced aggression in mice with adult rescue of the receptor, compared to vehicle injection, in number of pairs attacking (Figure S4H; χ21=9.60, p<0.01) and number of attacks (Figure S4I; F1,14=16.9, p<0.01). There was no significant reduction in aggression in CP-94,253-treated β-actin-tTS+ mice without rescue of 5-HT1BR expression (F1,14=0.121, p>0.05), suggesting that the effect of CP-94,253 on aggression was specific to 5-HT1BRs. These data demonstrate that the adult-rescued 5-HT1BRs are functional, and also highlight that there are distinct adult and developmental effects of 5-HT1BR on mouse aggression.
Early postnatal rescue of 5-HT1BR expression was able to reverse the aggressive phenotype in β-actin-tTS+ mice. Administration of doxycycline beginning at P0, but not at P7, prevented the development of adult aggression (Figure 3B). Specifically, following administration of doxycycline beginning at P0, there was no significant difference between genotypes in the percent of pairs showing aggression (χ21=0.41, p>0.05) nor in the time spent engaging in aggressive behaviors (Figure 3C; F1,8=3.23, p>0.05). Conversely, when doxycycline administration began at P7, β-actin-tTS+ mice showed elevated aggression compared to β-actin-tTS- controls (Fig 3B; χ21=6.20, p<0.05 for P7). Since rescue of receptor expression led to normal expression levels by 21d after the start of doxycycline administration (Figure S3C), these data suggest a sensitive period of approximately P21–28 for the development of aggressive behavior.
Further localizing the 5-HT1BR subpopulation(s) subserving aggressive behavior, we found that knockdown of postnatal forebrain heteroreceptors using CaMKII-tTS+ mice resulted in increased aggressive behavior. Specifically, more CaMKII-tTS+ pairs were aggressive compared to CaMKII-tTS- pairs (Figure 3D; 11 of 14 for tTS+ vs. 3 of 11 for tTS-; χ21=6.58, p<0.05). Additionally, CaMKII-tTS+ mice spent more time on average engaging in aggressive behavior–consisting mostly of tail rattling and biting attacks (Figure 3E and Figure S4C; F1,23=7.60, p<0.05)–and also had a shorter latency to attack (Figure S4D; χ21=13.44, p<0.001). Whole life treatment with doxycycline reduced the aggression in CaMKII-tTS+ mice to control levels (Figure 3D; χ21=0.42, p>0.05), and lengthened the latency to attack (Figure S4D; χ21=0.25, p>0.05). The time course of the CaMKII-tTS-mediated knockdown (see Figure S3C) was consistent with the sensitive period suggested from the β-actin-tTS studies above. Specifically, the majority of forebrain heteroreceptor knockdown in CaMKII-tTS+ mice was achieved by PN21, with the full extent of knockdown by PN28. Taken together, these data suggest that expression of forebrain heteroreceptors during the pubertal period is important for the development of male aggression.
5-HT1B autoreceptor knockdown in ePet-Cre+ mice did not significantly affect aggressive behavior. While baseline aggression was higher in this strain resulting in 5 out of 8 pairs showing aggressive behavior in the control ePet-Cre- mice, they were not significantly different from ePet-Cre+ mice (Figure 3F; χ21=0.58, p>0.05). There were also no significant differences between control ePet-Cre+::tetO1B−/− mice compared to ePet-Cre-::tetO1B−/− littermates (Figure 3F; χ21=0.25, p>0.05), ruling out effects of the transgene insertion on aggression in this paradigm. Additionally, there were no significant differences in the time spent engaging in aggressive behaviors (Figure 3G; F1,13=0.45, p>0.05), number of attacks (Figure S4E; F1,13=1.88, p>0.05), or latency to attack (Figure S4F; χ21=1.79, p>0.05).
Impulsive behavior in the DRL is mediated by adult expression of 5-HT1BRs
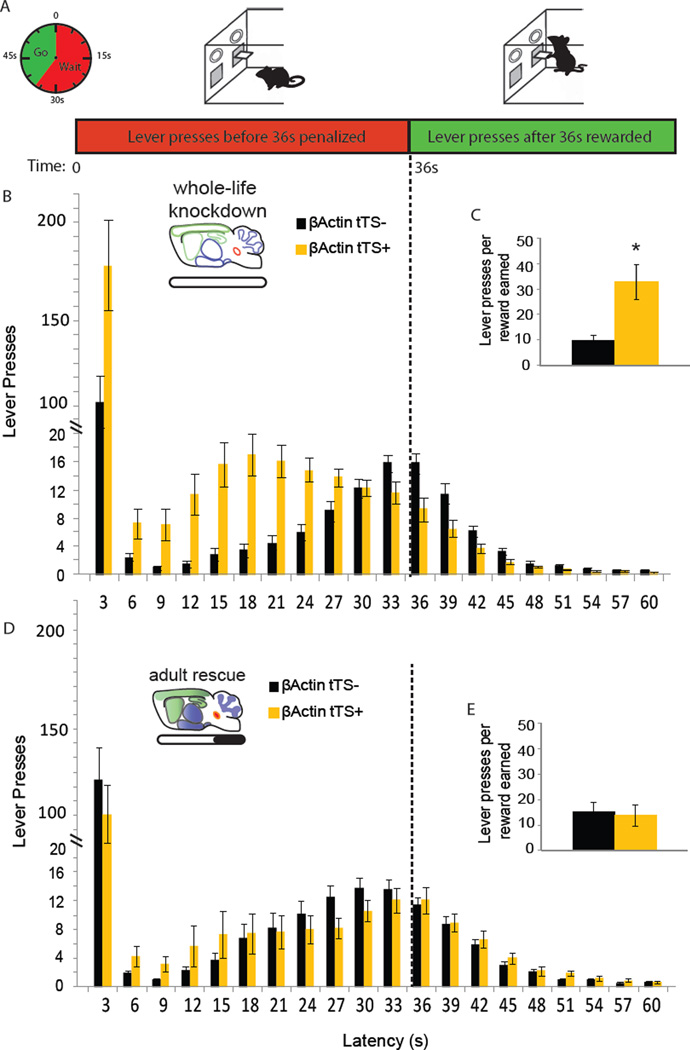
Two operant behavior paradigms were used to measure two aspects of impulsivity. The first, a differential reinforcement of low-rate responding (DRL) paradigm, reinforced the ability of a mouse to refrain from responding for a set time period. For example, in a DRL-36 paradigm, mice only receive a reward following a lever press if the lever press was preceded by 36 sec of no lever pressing (Figure 4A). Whole-brain knockout of the 5-HT1BR in β-actin-tTS+ resulted in deficits in the DRL task, indicating a more impulsive phenotype, as seen in the constitutive 5-HT1BR knockout (Pattij et al., 2003). Compared to littermate β-actin-tTS- controls, β-actin-tTS+ mice had 80% more burst responses, which are responses with a latency of less than 3 sec (Figure 4B; F1,28=7.2, p<0.05). The latencies of the remaining non-burst responses represented a unimodal distribution which was significantly different between genotypes (Figure 4B; F1, 28=23.63, p<0.05). The distribution was left-shifted in β-actin-tTS+ mice, indicating increased premature responding (genotype × latency interaction: F18, 504=11.79, p<0.05). Cumulative distribution functions of individual animals revealed that the majority of responses from β-actin-tTS+ mice were made earlier than that of their littermate tTS- controls (Figure S5C). This resulted in an increased number of non-rewarded lever presses, and therefore a significantly higher response-to-reward ratio in β-actin-tTS+ mice (Figure 4C; F1, 28=11.89, p<0.05). Finally, a Gaussian function was fitted to the non-burst response distribution and revealed a significantly earlier peak responding latency in β-actin-tTS+ mice compared to β-actin-tTS- controls, whose peak responding was nearly 10 sec later (Figure S5D; F1,28=23.04, p<0.05). The poor performance of the β-actin-tTS+ mice in the DRL paradigm was not likely due to a lack of learning, since the peak of the response distribution increased as the target wait time was increased during training (Figure S5B). In fact, the number of sessions required to meet criterion in lever pressing acquisition was lower in β-actin-tTS+ mice, suggesting no deficit in overall operant learning (F1,28=4.91, p<0.05). Additionally, there were no significant differences in the number of head entries into the reward receptacle (Figure S5E; F1,28=1.81, p>0.05). A control experiment ruled out an effect of the tTS transgene insertion on DRL behavior, as the behavior of β-actin-tTS+ mice treated with doxycycline throughout life was not significantly different from that of littermate control β-actin-tTS- mice (Figure S5K–P). Overall, these data confirmed a role for 5-HT1BRs in regulating impulsivity.
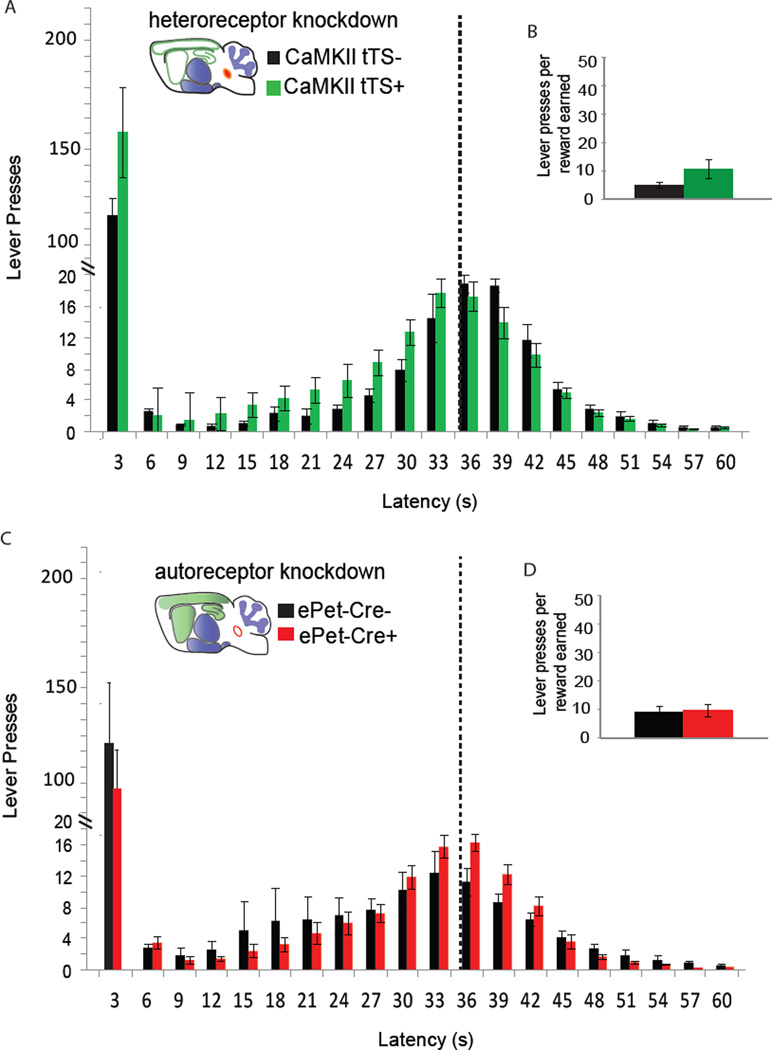
Figure 4. Adult expression of 5-HT1BR mediates impulsive behavior in the DRL paradigm.
A) Schematic describing the DRL operant paradigm. B, D) The distribution of the latencies of lever presses in the DRL is shown for β-actin-tTS+ mice (yellow) and their littermate β-actin-tTS- controls (black) for whole-life knockdown (B) and adult rescue (D) groups. Group means ± SEM are presented for latencies binned in 3 sec intervals. The dotted line represents the minimum response latency (36 sec) which is rewarded. C, E) The average ratio of the number of lever presses to the number of rewards earned ± SEM is shown for whole-life knockdown (C) and adult rescue (E) groups. *, p<0.05. See also Figure S5.
Interestingly and unexpectedly, the impulsive phenotype was fully recovered with adult rescue of 5-HT1BR, differentiating it from the aggressive phenotype. Doxycycline treatment beginning in adulthood (P60) resulted in no significant genotype differences between β-actin-tTS- and β-actin-tTS+ mice in any parameter analyzed from the DRL paradigm (Figure S5F–J). Specifically, burst and non-burst responding in β-actin-tTS+ mice were normalized to control levels (Figure 4D; F1,26=0.61, p>0.05), and the distribution of non-burst responses was not significantly different from β-actin-tTS- controls (F1,26=0.01, p>0.05). The cumulative distribution functions of individual animals were overlapping (Figure S5H), and there were no significant differences between genotypes in the ratio of lever presses to reward earned (Figure 4E; F1,26=0.05, p>0.05). The means of the fitted curve were not significantly different for β-actin-tTS- and β-actin-tTS+ mice treated with doxycycline (Figure S5I; F1,26=0.02, p>0.05). Taken together with the data from the aggression assays, adult rescue of 5-HT1BRs resulted in aggressive, but not impulsive mice. This dissociates the impulsive phenotype from that of aggression and suggests different sensitive periods of serotonergic modulation.
The contribution of 5-HT1B autoreceptors and forebrain heteroreceptors to the impulsive phenotype was also assessed in the DRL operant paradigm. Neither CaMKII-tTS+ nor ePet-Cre+ mice were more impulsive than their littermate controls (Figure 5 and Figure S6A–J). There were no significant genotype differences in the number of burst lever presses (Figure 5A, F1,11=1.82, p>0.05 for CaMKII-tTS; Figure 5C, F1,16=0.93, p>0.05 for ePet-Cre), or in the distribution of lever press latencies (F1,11=2.48, p>0.05 for CaMKII-tTS; F1,16=0.34, p>0.05 for ePet-Cre). The cumulative distribution functions of individual animals showed largely overlapping response curves for both CaMKII-tTS+ and ePet-Cre+ compared to their littermate controls (Figures S6C and S6H). Lastly, there were no significant differences in the ratio of lever presses to rewards earned (Figure 5B, F1,11=3.21, p>0.05 for CaMKII-tTS; Figure 5D, F1,16=0.06, p>0.05 for ePet-Cre), nor in the mean of the curve fitted to lever press response distributions (Figure S6D and S6I; F1,11=2.18, p>0.05 for CaMKII-tTS; F1,16=0.10, p>0.05 for ePet-Cre). Control experiments comparing ePet-Cre+::tetO1B−/− mice to ePet-Cre-::tetO1B−/− mice, and CaMKII-tTS+ mice treated with doxycycline throughout life to CaMKII-tTS- mice treated with doxycycline throughout life, showed no significant effects of insertion of either transgene on behavior in the DRL (Figure S6K–V). Compared to the results from the aggression studies, the data again suggests a dissociation of aggressive and impulsive behaviors, with forebrain heteroreceptor knockdown resulting in increased aggression, but not impulsivity.
Figure 5. Neither CaMKII-tTS+ nor ePet-Cre+ mice show impulsive behavior in the DRL paradigm.
A, C) The distribution of the latencies of lever presses in the DRL is shown for CaMKII-tTS+ mice (green), ePet-Cre+ mice (red) and their respective littermate controls (black). Group means ± SEM are presented for latencies binned in 3 sec intervals. The dotted line represents the minimum response latency (36 sec) which is rewarded. B, D) The average ratio of the number of lever presses to the number of rewards earned ± SEM is shown for each group. See also Figure S6.
Impulsive behavior in the Go/No-Go task is mediated by adult expression of 5-HT1BRs
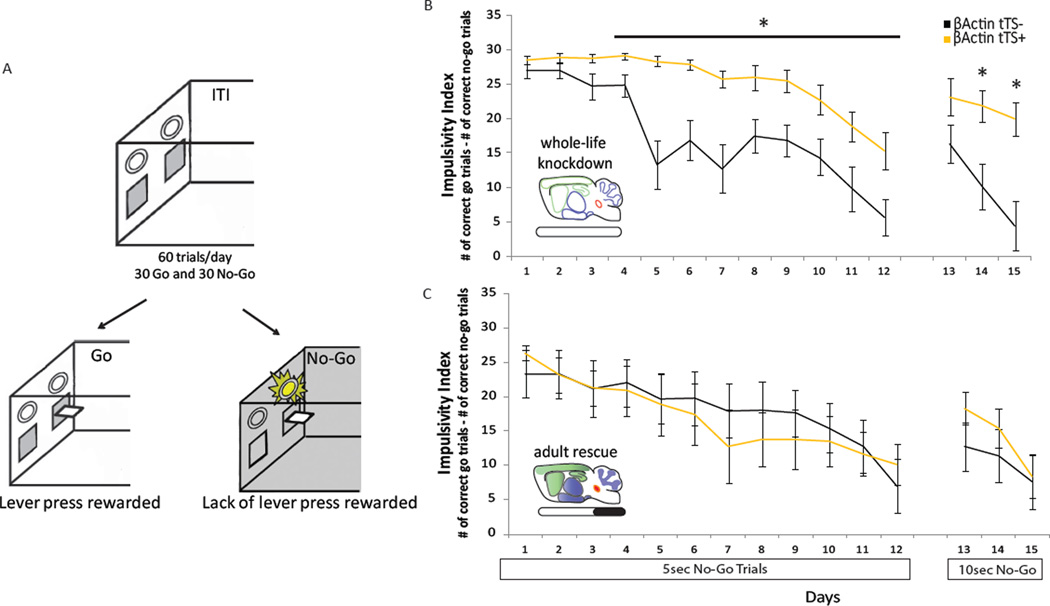
To extend the behavioral differences seen in the DRL paradigm, a second operant behavioral task measuring impulsivity was used. A mouse model of the Go/No-Go paradigm adapted from previous studies (McDonald et al., 1998) rewarded the ability to refrain from responding during specific trials. Mice were presented with ‘Go’ trials, in which lever presses were rewarded, and ‘No-Go’ trials, in which an absence of lever pressing was rewarded (Figure 6A). β-actin-tTS+ mice were more impulsive than β-actin-tTS- littermate controls, and the behavioral deficit was ameliorated with doxycycline treatment in adulthood. Specifically, in the absence of doxycycline, β-actin-tTS+ mice were more impulsive across all trials compared to β-actin-tTS- controls as indexed by the number of correct No-Go trials subtracted from the number of correct Go trials (Figure 6B, F1,27=14.29, p<0.001). Broken down by trial type, β-actin-tTS+ mice earned more rewards during Go trials (Figure S7A; F1,27=7.75, p<0.01), and fewer rewards during No-Go trials (Figure S7B, F1,27=11.63, p<0.005) compared to β-actin-tTS- mice. Over the 12 sessions, while both genotypes decreased their impulsivity index, the decrease was greater in β-actin-tTS- mice (genotype × session interaction: F11,297=3.03, p<0.001). During the 12th session, β-actin-tTS+ had significantly more false alarms (unrewarded No-Go trials), averaging over 60%, compared to β-actin-tTS- mice, who had a 36% false alarm rate (F1,27=5.58, p<0.05). Increasing the duration of the No-Go trials from 5 sec to 10 sec resulted in decreases in the performance of both genotypes, as measured by impulsivity index. However, β-actin-tTS- mice were able to recover their performance within 3 days, while β-actin-tTS+ mice were not (Figure 6B; F2,54=4.65, p<0.05). These deficits in the performance of β-actin-tTS+ mice in the Go/No-Go task suggested an impulsive phenotype, consistent with results from the DRL paradigm.
Figure 6. Adult expression of 5-HT1BR mediates impulsive behavior in a Go/No-Go task.
A) Schematic illustrating the house-light and lever-light cues in the operant box during the inter-trial interval (ITI) and during Go and No-Go trials. B, C) The group means ± SEM of the impulsivity index, defined as the number of rewarded No-Go trials subtracted from the number of rewarded Go trials, is shown over 15 days for β-actin-tTS- (black) and tTS+ (yellow) mice in the absence of doxycycline (B) or following adult administration (C). No-Go trials lasted for 5 sec on the first 12 days, and 10 sec on days 13–15. *, p<0.05. See also Figure S7.
To confirm that the poor performance seen in the β-actin-tTS+ mice in the Go/No-Go task was due to an impulsive phenotype rather than a deficit in the ability to learn the trial cues, a control experiment assessed cue-based learning. In this task, the cues that signaled Go and No-Go trials were used to signal left-lever or right-lever trials, and as such, all trials required a response (Figure S7C). The number of correct trials, represented by the number of rewards earned, was not different between genotypes (Figure S7D; F1,27=2.27, p>0.05). This suggests that the inability to withhold responses during No-Go trials was not due to a deficit in cue-learning.
Rescue of 5-HT1BR expression in adulthood with administration of doxycycline resulted in no significant differences in performance in the Go/No-Go task between β-actin-tTS+ mice and β-actin-tTS-controls. Specifically, there were no significant differences in impulsivity index (Figure 6C; F1,27=0.10, p>0.05), number of correct Go trials (F1,27=0.01, p>0.05), or number of correct No-Go trials (F1,27=0.30, p>0.05). The number of false alarms on day 12 was also not significantly different between genotypes (F1,27=0.01, p>0.05). These results were consistent with performance seen in the DRL, suggesting that adult expression of 5-HT1BR modulates impulsivity as measured in two operant assays of behavioral inhibition.
Adult 5-HT1BRs regulate dopamine levels in the nucleus accumbens
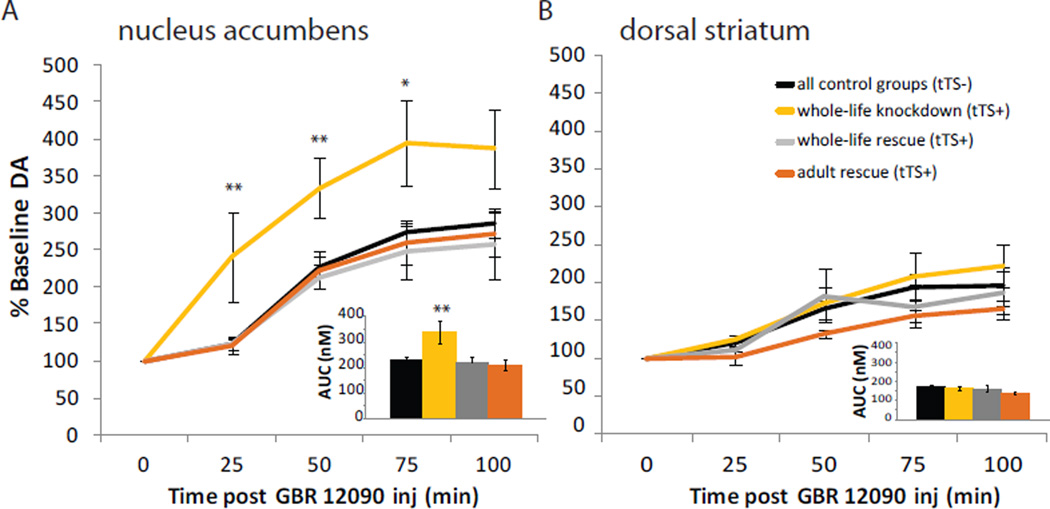
Given the importance of dopamine in modulating impulsive behavior (e.g. Cole and Robbins, 1989; Pattij et al., 2007), we measured dopamine (DA) levels in our knockdown mice. To determine the effects of 5-HT1BR expression on CNS DA levels, microdialysis was performed in the nucleus accumbens (NAc) and dorsal striatum following peripheral injection of GBR 12090, a DA transporter (DAT) blocker (Figure S8C,D). There was a main effect of 5-HT1BR expression on DA levels in the NAc (Figure 7A; F3,32=5.18, p<0.01). β-actin-tTS+ mice had significantly greater increases in DA levels in the NAc compared to β-actin-tTS- mice (F1,20=9.71, p<0.01). Interestingly, adult rescue of the 5-HT1BR normalized DA levels in the NAc of β-actin-tTS+ mice. Specifically, adult treatment with doxycycline in β-actin-tTS+ mice resulted in significantly smaller GBR 12090-induced DA increases compared to β-actin-tTS+ mice without doxycycline (F1,13=7.05, p<0.05); the DA increases in β-actin-tTS+ mice were not significantly different from that of β-actin-tTS- mice or β-actin-tTS+ mice given doxycycline throughout life (F1,21=0.55, p>0.05 and F1,12=0.06, p>0.05, respectively). These results suggest that adult expression of 5-HT1BR regulates NAc DA levels. In contrast, there were no significant effects of 5-HT1BR expression on levels of DA in the dorsal striatum, as previously reported (Shippenberg et al., 2000; Figure 7B; F3,32=1.53, p>0.05). There were also no group differences in the baseline levels of DA in the NAc or dorsal striatum (Figure S8A, NAc: F3,32=1.60, p>0.05; S8B, dorsal striatum: F3,32=1.66, p>0.05). These data indicate that 5-HT1BRs regulate DA release in the NAc during adulthood, which may be a mechanism by which 5-HT1BRs affect impulsivity.
Figure 7. Adult expression of 5-HT1BR mediates dopamine levels in the nucleus accumbens.
Dopamine levels in microdialysis samples from the nucleus accumbens (A) and dorsal striatum (B) are shown as group means ± SEM of the percent of baseline for 100 min following peripheral injection of the dopamine transporter inhibitor, GBR 12090. Insets show area under the curve (AUC) of the dopamine concentrations (nM) over the 100 minutes following injection of GBR 12090. *, p<0.05; **, p<0.01. See also Figure S8.
Discussion
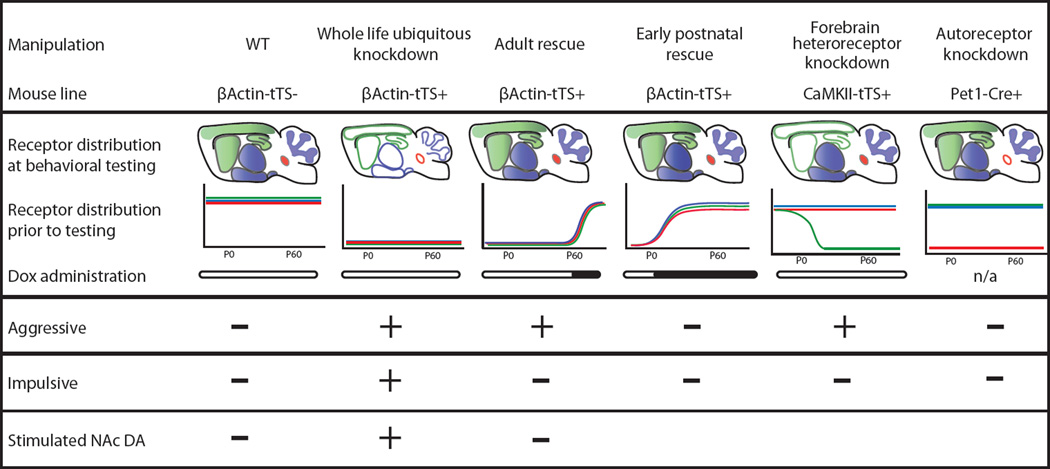
The development of a tissue-specific and temporally conditional 5-HT1BR mouse model allowed us to investigate the contribution of serotonin to the neural circuits underlying aggression and impulsivity (Figure 8). First, we showed that aggressive behavior is mediated by the developmental expression of 5-HT1B heteroreceptors. Specifically, whole-life, whole-brain knockdown of 5-HT1BRs resulted in increased aggressive behavior, in agreement with our previous results using constitutive knockouts (Saudou et al., 1994). Consistent with the presence of a developmental sensitive period, rescue of 5-HT1BR expression in early post-natal development, but not in adulthood, ameliorated the aggressive behavior. Furthermore, forebrain heteroreceptors located on CaMKII-expressing cells mediated this aggressive phenotype, while a reduction in autoreceptors did not modulate aggression. In contrast, the data show a different pattern of results for the modulation of impulsive behavior. Using two operant conditioning paradigms (DRL and Go/No-Go), mice which lacked all 5-HT1BRs were unable to withhold lever pressing responses, suggesting heightened impulsivity. Interestingly, this impulsive phenotype was fully reversed by rescue of receptor expression in adulthood. Similar to aggression, knockdown of 5-HT1B autoreceptors did not affect impulsive behavior. However, unlike in aggression, forebrain 5-HT1BRs located in CaMKII-expressing cells did not mediate impulsive behavior. Since CaMKII-tTS+ cells represent only a subset of 5-HT1B heteroreceptor-containing neurons, these data suggest that a different set of 5-HT1B receptors, located on either non-CaMKII+ neurons, neurons in regions not knocked down by our CaMKII-tTS line (e.g. lateral septum, hypothalamus, VTA), or even non-neuronal cells are involved in modulating impulsivity compared to aggressive behavior. Overall, the data presented here support the claim that serotonin can affect aggression and impulsivity through distinct circuits and during different time periods by acting through 5-HT1BRs.
Figure 8.
Summary of spatial and temporal contributions of 5-HT1BR to aggression, impulsivity and dopamine levels.
We have discovered a developmental sensitive period during which serotonin affects later-life adult aggressive behavior. Many studies in both humans and animal models now support the idea that there are specific periods of development during which genetic and environmental perturbations can influence adult phenotypes related to psychiatric disorders, including anxiety, depression, aggression and antisocial personality disorder (Ansorge et al., 2004; Caspi et al., 2002; Caspi et al., 2003; Dodge et al., 1990; Richardson-Jones et al., 2011). Aggressive behavior has been linked to developmental alterations in monoamine levels through disruptions in MAOA activity. A genetic null mutation in the gene encoding MAOA results in increased aggressive and antisocial behavior (Brunner et al., 1993). A less severe reduction in MAOA activity caused by a variable number tandem repeat polymorphism in the MAOA promoter is associated with conduct disorder and antisocial behavior when coupled with childhood maltreatment (Caspi et al., 2002). Lastly, an MAOA KO mouse model displays increased aggressive behavior, which correlates with altered developmental levels of serotonin and norepinephrine (Cases et al., 1995). Other recent studies have implicated dopaminergic signaling during development in the generation of aggressive behavior in rodents. Specifically, blockade of dopamine reuptake in mice during a postnatal period (P22-P41) resulted in increased adult aggression (Yu et al., 2014). Furthermore, aggression in hamsters induced by adolescent administration of cocaine was ameliorated by co-administration of paliperidone, a D2/5-HT2A receptor antagonist (Schwartzer et al., 2009). This study implicates the middle of adolescence as the sensitive period for dopaminergic and serotonergic effects on aggression. The sensitive periods reported in these studies are consistent with the sensitive period that we approximate from our data. Both the time course of the doxycycline studies in β-actin mice (taking into account the 21 day lag in complete rescue of receptor expression; see Figure S3A), and the developmental time course of CaMKII promoter activity (see Figure S3C), suggest that the period encompassing postnatal days P21–28 is important for the maturation of neural circuits underlying adult aggressive behavior. However, given the slow kinetics of the doxycycline effect on receptor expression, other tools will be required to accurately define this sensitive period further. This proposed sensitive period corresponds with the onset of adolescence, which is also the onset of play behavior, the development of social hierarchies, and the appearance of aggressive behavior (Bartolomucci et al., 2004; Pellis and Pasztor, 1999; Terranova et al., 1998).
The developmental, rather than adult, mechanism of action of 5-HT1BRs on aggression shown here, is dissociated from pharmacological data showing that activation of 5-HT1BRs with agonists such as the serenics reduces aggressive behavior when administered in adulthood (Mos et al., 1992; Sijbesma et al., 1991). The disconnect between knockout and pharmacological phenotypes has been previously identified with regards to 5-HT1BR signaling (Castanon et al., 2000), but the lack of conditional knockouts prevented investigation into compensatory and/or developmental contributions to these effects. Here, we show a developmental contribution of the receptor to adult aggressive behavior, which seems to be distinct from the 5-HT1BR-mediated pharmacological effects on adult aggression. This dissociation is suggested by the fact that aggression in the adult rescue mice could still be attenuated with acute administration of a 5-HT1BR agonist. One possible explanation is that there could be two populations of 5-HT1BRs that modulate aggression circuits at different developmental time points. Also, although we show here that knocking out 5-HT1BRs in CaMKII+ cells is sufficient to generate an aggressive phenotype, it is still possible that other 5-HT1BRs are involved. Specifically, 5-HT1BRs are located in a number of other brain regions implicated in aggression including the periaqueductal grey, ventromedial hypothalamus, and lateral septum (Lin et al., 2011; Siegel et al., 1999; Simon et al., 1998). Adult expression of one of these populations of receptors may also modulate aggressive behavior. Another possibility is that serenics are affecting aggression in adulthood indirectly through another mechanism, such as reducing impulsivity. Finally, our data do not rule out the possibility that 5-HT1BRs affect different facets of aggressive behavior at different time points. Specifically, our paired aggression assay could be measuring aggression-eliciting factors, rather than or in addition to offensive aggressive behavior directly.
Unlike aggressive behavior, impulsive behavior is mediated by the expression of 5-HT1BRs during adulthood. This was not predicted given the impulsive aggression seen in the 5-HT1BR KOs, and the frequent co-occurrence of these behavioral phenotypes. Specifically, using the DRL and Go/No-go paradigms, we measured impulsive action, a concept which includes behavioral inhibition, and is characterized by the inability to inhibit a response. This type of impulsivity may be dissociated from impulsive choice, which represents intolerance to delays and is frequently measured in delayed discounting tasks (Bari and Robbins, 2013; Broos et al., 2012). Evidence suggests that 5-HT1BRs affect impulsive action (Pattij et al., 2003), but may not affect impulsive choice (Brunner and Hen, 1997). This is important because these two aspects of impulsivity are thought to be mediated by distinct circuits, and may contribute to different psychiatric disorders (Dalley et al., 2011; Robbins et al., 2012). For example, deficits in impulsive action predict substance abuse potential and severity of pathological gambling (Brevers et al., 2012; Loos et al., 2009), while deficits in impulsive choice may play a larger role in ADHD (Solanto et al., 2001; Sonuga-Barke, 2003). Interestingly, studies have implicated 5-HT1BR expression levels and polymorphisms in substance use disorders and pathological gambling (Cao et al., 2013; Potenza et al., 2013; Proudnikov et al., 2006; Rocha et al., 1998). Our findings suggest that different pharmacological approaches may be necessary depending on the type of inhibitory control or impulsivity (or impulsivities) associated with each disorder.
The relationship between serotonin signaling and impulsive behavior is complex. On one hand, studies show that impulsive behavior is negatively correlated with serotonin levels (Fairbanks et al., 2001; Harrison et al., 1997), and activation of serotonin neurons or serotonin receptors reduces impulsivity (Evenden, 1999; Miyazaki et al., 2014). However, other evidence suggests that increased serotonin levels in the prefrontal cortex are associated with increases in impulsivity (Dalley et al., 2002), suggesting potential brain region-specific effects. Additionally, activation of different serotonin receptors can have opposing effects on impulsive behavior (Winstanley et al., 2004). Finally recent evidence suggests that dorsal raphe serotonin neurons also release glutamate which can have behavioral effects similar to that of serotonin (Liu et al., 2014). Although 5-HT1BR knockouts have increased serotonin levels (Gardier et al., 2003; Malagie et al., 2001), it remains unclear whether this change in serotonin signaling plays a role in modulating impulsivity. Here, we show that knocking down 5-HT1B autoreceptors is not sufficient to change impulsive behavior; however, our significant but incomplete knockdown still leaves open the possibility for the remaining autoreceptors to be involved in the behavioral effects of 5-HT1BR knockout. Another possibility is that changes in serotonergic tone via 5-HT1B autoreceptors contribute to increased impulsivity only in conjunction with the absence of certain 5-HT1BR heteroreceptors. In such a situation, knockdown of both autoreceptors and another population of 5-HT1B heteroreceptors would be necessary to elicit increased impulsive behavior. One potential scenario leading to impulsivity could therefore be an interaction of increased serotonergic tone coupled with increased dopamine levels, as is supported by the fact that depletion of serotonin with 5,7-dihydroxytryptamine results in an attenuation of amphetamine-induced premature responding (Harrison et al., 1997). Similarly, serotonin signaling could interact with dopamine to affect impulsivity.
Consistent with our results, there is evidence to support a role for NAc DA levels in the regulation of impulsive action. Local injection of amphetamine into the NAc increases premature responding, suggesting a lack of behavioral inhibition or increased impulsive action (Cole and Robbins, 1987). These effects could be blocked by a D2 receptor antagonist, and were not affected by lesions of the norepinephrine system, suggesting a dopaminergic action of amphetamine on impulsivity (Pattij et al., 2007). Although 5-HT1BR may affect impulsive action via dopamine in the NAc, there are many other potential mechanisms for these effects. Dopamine signaling in a number of brain regions, not limited to the NAc, has been linked to impulsivity, and could be involved in 5-HT1BR modulation of impulsive action. For example, levels of dopamine and dopamine receptors in the PFC are correlated with increased impulsive action (Bari and Robbins, 2013; Counotte et al., 2009; Moon et al., 2014). Additionally, other neurotransmitters and neuromodulators play an important role in modulating impulsive action. For example, increasing norepinephrine levels results in increased impulsivity (Swann et al., 2005; Swann et al., 2013) and increased levels of both vasopressin and neuropeptide Y are associated with increased impulsive behavior (Aliczki et al., 2014; Coccaro et al., 2012). Furthermore, even decreased GABA synthesis in the NAc core is sufficient to cause increases in impulsive action (Caprioli et al., 2014).
Overall, this mouse model of 5-HT1BR-associated deficits in impulse control provides a useful tool for the discovery of new treatments for psychiatric disorders in which impulsivity is a key phenotype. There is a substantial amount of evidence linking 5-HT1BR to impulsivity in humans, and our findings suggest that pharmacologic interventions targeting 5-HT1BR-mediated impulsivity may be effective in adults. Recent haplotype and meta-analyses have confirmed links between 5-HT1BR polymorphisms and heroin, cocaine, and alcohol abuse (Cao et al., 2013; Proudnikov et al., 2006). Additionally, increased 5-HT1BR binding is correlated with severity of gambling in pathological gamblers (Potenza et al., 2013). Lastly, a number of studies including a meta-analysis have found significant associations between 5-HT1BR polymorphisms and ADHD in a number of different populations (Banerjee et al., 2012; Gizer et al., 2009). Future research will be aimed at investigating the circuits underlying these effects of 5-HT1BR on impulsivity, and will hopefully set the stage for pharmacological interventions aimed at decreasing impulsive behavior in disorders such as substance abuse, pathological gambling, and ADHD.
Experimental Procedures
Please see Supplemental Experimental Procedures for full details of methods and reagents.
Mice
A vector containing a floxed-tetO-htr1b cDNA-PGK-neo cassette was introduced into the genome through homologous recombination in place of the endogenous htr1b gene coding region (Figure S1). Spatial- and temporal-specific knockdown was achieved through genetic crosses to β-actin-tTS, CaMKII-tTS, and ePet-Cre mice, and by administration of doxycycline to tTS lines. All animal care and testing was approved by the Institutional Animal Use and Care Committee and was in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals.
Tissue
For receptor binding and in situ hybridization analysis, mice were sacrificed by cervical dislocation and decapitation. 125I-cyanopindolol autoradiography for 5-HT1BR localization was performed based on a previously described protocol (Saudou et al., 1994). In situ hybridization was performed based on the Allen Brain Atlas Protocol using a digoxigenin (DIG)-labed htr1b cRNA probe previously described (Tanaka et al., 2012).
Behavior
Aggression assays were conducted on male mice at 13–15 weeks of age, using a paired housing procedure in which two mice of the same genotype are co-housed separated by a plexiglass divider, as previously described (Yu et al., 2014). Operant conditioning studies beginning at 12 weeks of age were conducted in eight identical operant chambers (Med Associates Inc., St Albans, VT) on food-restricted mice. Following behavioral shaping, DRL and Go/No-Go paradigms were used to test impulsive action.
In vivo microdialysis
Samples were collected from the dorsal striatum and nucleus accumbens brain regions of awake and freely moving mice, at baseline conditions and following injection of GBR 12090 or escitalopram. Dialysates were analyzed for DA or 5-HT levels using HPLC.
Supplementary Material
Highlights.
Mouse model developed for tissue-specific and inducible knockdown of 5-HT1BR
5-HT1BR affects aggression and impulsivity through distinct mechanisms.
Developmental 5-HT1B heteroreceptors determine adult aggressive behavior.
Adult expression of 5-HT1B heteroreceptors modulates impulsive behavior.
Acknowledgements
The authors would like to thank Drs. Peter Balsam, Mazen Khierbek, and Melody Wu for comments on previous versions of this manuscript, Brian Bulthuis for assistance with data analysis, and Jenny Payne for technical assistance. Facilities and equipment of the Rodent Neurobehavioral Assessment Core at the New York State Psychiatric Institute were used for behavioral testing. Funding provided by F32 MH100888 to KMN, Early Stage Investigator Award from the National Center for Responsible Gaming to KMN, T32 MH015174 to KMN, R01 MH082773 to CB, K08 MH087718 to SEA, Gerstner Scholar Grant to SEA, Paul Janssen Translational Research Fellowship to SEA, Burroughs Wellcome CAMS Award to SEA, R01 MH083862 to RH, and R37 MH068542 to RH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliczki M, Fodor A, Balogh Z, Haller J, Zelena D. The effects of lactation on impulsive behavior in vasopressin-deficient Brattleboro rats. Hormones and behavior. 2014;66:545–551. doi: 10.1016/j.yhbeh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Audero E, Mlinar B, Baccini G, Skachokova ZK, Corradetti R, Gross C. Suppression of serotonin neuron firing increases aggression in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8678–8688. doi: 10.1523/JNEUROSCI.2067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Banerjee E, Banerjee D, Chatterjee A, Sinha S, Nandagopal K. Selective maternal inheritance of risk alleles and genetic interaction between serotonin receptor-1B (5-HTR1B) and serotonin transporter (SLC6A4) in ADHD. Psychiatry research. 2012;200:1083–1085. doi: 10.1016/j.psychres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Chirieleison A, Gioiosa L, Ceresini G, Parmigiani S, Palanza P. Age at group formation alters behavior and physiology in male but not female CD-1 mice. Physiology & behavior. 2004;82:425–434. doi: 10.1016/j.physbeh.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Verbruggen F, Bechara A, Kornreich C, Verbanck P, Noel X. Impulsive action but not impulsive choice determines problem gambling severity. PloS one. 2012;7:e50647. doi: 10.1371/journal.pone.0050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, Weierink L, Ham J, de Geus EJ, Schoffelmeer AN, et al. The relationship between impulsive choice and impulsive action: a cross-species translational study. PloS one. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry research. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Annals of the New York Academy of Sciences. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Cao J, LaRocque E, Li D. Associations of the 5-hydroxytryptamine (serotonin) receptor 1B gene (HTR1B) with alcohol, cocaine, and heroin abuse. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2013;162B:169–176. doi: 10.1002/ajmg.b.32128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, Voon V, Carpenter TA, Everitt BJ, Robbins TW, et al. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biological psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacology, biochemistry, and behavior. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. International clinical psychopharmacology. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Liu T, Mathe AA. Cerebrospinal fluid neuropeptide Y-like immunoreactivity correlates with impulsive aggression in human subjects. Biological psychiatry. 2012;72:997–1003. doi: 10.1016/j.biopsych.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behavioural brain research. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Conner TS, Jensen KP, Tennen H, Furneaux HM, Kranzler HR, Covault J. Functional polymorphisms in the serotonin 1B receptor gene (HTR1B) predict self-reported anger and hostility among young men. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2010;153B:67–78. doi: 10.1002/ajmg.b.30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Robbins TW. Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11993–11999. doi: 10.1523/JNEUROSCI.2513-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Dennis RL, Lay DC, Jr, Cheng HW. Effects of early serotonin programming on behavior and central monoamine concentrations in an avian model. Behavioural brain research. 2013;253:290–296. doi: 10.1016/j.bbr.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats VII: the effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999;146:422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Garcia-Forero C, Gallardo-Pujol D, Maydeu-Olivares A, Andres-Pueyo A. Disentangling impulsiveness, aggressiveness and impulsive aggression: an empirical approach using self-report measures. Psychiatry research. 2009;168:40–49. doi: 10.1016/j.psychres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Gardier AM, David DJ, Jego G, Przybylski C, Jacquot C, Durier S, Gruwez B, Douvier E, Beauverie P, Poisson N, et al. Effects of chronic paroxetine treatment on dialysate serotonin in 5-HT1B receptor knockout mice. Journal of neurochemistry. 2003;86:13–24. doi: 10.1046/j.1471-4159.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Annals of the New York Academy of Sciences. 1997;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Molecular psychiatry. 2009;14:381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, van der Sluis S, Bochdanovits Z, van Zutphen IJ, Pattij T, Stiedl O, Neuro BMPc, Smit AB, Spijker S. Activity and impulsive action are controlled by different genetic and environmental factors. Genes, brain, and behavior. 2009;8:817–828. doi: 10.1111/j.1601-183X.2009.00528.x. [DOI] [PubMed] [Google Scholar]
- Malagie I, Trillat AC, Bourin M, Jacquot C, Hen R, Gardier AM. 5-HT1B Autoreceptors limit the effects of selective serotonin re-uptake inhibitors in mouse hippocampus and frontal cortex. Journal of neurochemistry. 2001;76:865–871. doi: 10.1046/j.1471-4159.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- Mallo M, Kanzler B, Ohnemus S. Reversible gene inactivation in the mouse. Genomics. 2003;81:356–360. doi: 10.1016/s0888-7543(03)00032-6. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learning & memory. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Current biology : CB. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Hori T, Takahashi T. 5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. The European journal of neuroscience. 2006;24:1946–1954. doi: 10.1111/j.1460-9568.2006.05063.x. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Kim CJ, Lee YJ, Hong M, Han J, Bahn GH. Effect of atomoxetine on hyperactivity in an animal model of attention-deficit/hyperactivity disorder (ADHD) PloS one. 2014;9:e108918. doi: 10.1371/journal.pone.0108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, van Aken H. The effects of intraventricular administration of eltoprazine, 1-(3-trifluoromethylphenyl)piperazine hydrochloride and 8-hydroxy-2-(di-n-propylamino)tetralin on resident intruder aggression in the rat. European journal of pharmacology. 1992;212:295–298. doi: 10.1016/0014-2999(92)90348-8. [DOI] [PubMed] [Google Scholar]
- Pattij T, Broersen LM, van der Linde J, Groenink L, van der Gugten J, Maes RA, Olivier B. Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT1A and 5-HT1B receptor knockout mice. Behavioural brain research. 2003;141:137–145. doi: 10.1016/s0166-4328(02)00345-5. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Engel G, Palacios JM. beta-Adrenoceptor blocking agents recognize a subpopulation of serotonin receptors in brain. Brain research. 1985;343:403–408. doi: 10.1016/0006-8993(85)90766-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Developmental psychobiology. 1999;34:175–182. [PubMed] [Google Scholar]
- Potenza MN, Walderhaug E, Henry S, Gallezot JD, Planeta-Wilson B, Ropchan J, Neumeister A. Serotonin 1B receptor imaging in pathological gambling. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:139–145. doi: 10.3109/15622975.2011.598559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, Ott J, Kreek MJ. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenetics and genomics. 2006;16:25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Melloni RH., Jr Repeated fluoxetine administration during adolescence stimulates aggressive behavior and alters serotonin and vasopressin neural development in hamsters. Behavioral neuroscience. 2012;126:640–653. doi: 10.1037/a0029761. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends in cognitive sciences. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neuroscience and biobehavioral reviews. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Morrison RL, Ricci LA, Melloni RH., Jr Paliperidone suppresses the development of the aggressive phenotype in a developmentally sensitive animal model of escalated aggression. Psychopharmacology. 2009;203:653–663. doi: 10.1007/s00213-008-1412-4. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Hen R, He M. Region-specific enhancement of basal extracellular and cocaine-evoked dopamine levels following constitutive deletion of the Serotonin(1B) receptor. Journal of neurochemistry. 2000;75:258–265. doi: 10.1046/j.1471-4159.2000.0750258.x. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neuroscience and biobehavioral reviews. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacology, biochemistry, and behavior. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neuroscience and biobehavioral reviews. 1998;23:325–336. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of abnormal child psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and biobehavioral reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biological psychiatry. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG. Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology. 2013;229:83–94. doi: 10.1007/s00213-013-3088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KF, Samuels BA, Hen R. Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:2395–2401. doi: 10.1098/rstb.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. Journal of comparative psychology. 1998;112:3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Gingrich JA, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, Turecki G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2007;144B:996–1002. doi: 10.1002/ajmg.b.30521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.