Abstract
Mental stress induced left ventricular dysfunction (LVD) has been associated with a greater risk of adverse events in coronary heart disease (CHD) patients independent of conventional risk indicators. The underlying biochemical mechanisms of this cardiovascular condition are poorly understood. Our objective was to use metabolomics technology to identify biochemical changes that co-occur with mental stress-induced LVD in patients with clinically stable CHD. Participants were adult CHD patients who were recruited for mental stress-induced myocardial ischemia screening. For this study, we randomly selected 30 patients representing the extremes of the mental stress-induced left ventricular ejection fraction (LVEF) change distribution; 15 who showed LVD (i.e. LVEF reduction ≥5) and 15 who showed a normal left ventricular response (NLVR; i.e. a LVEF increase of ≥5) to three mental stressors. An electrochemistry based metabolomics platform was used to profile pre- and post-stress serum samples yielding data for 22 known compounds, primarily within the tyrosine, tryptophan, purine and methionine pathways. There were significant stress-induced changes in several compounds. A comparison between the NLVR and LVD groups showed significant effects for kynurenine (p = .036, N-acetylserotonin (p = .054), uric acid (p = .015), tyrosine (p = .019) and a trend for methionine (p = .065); the NLVR group showed a significantly greater stress-induced reduction in all of those compounds compared to the LVD group. Many of these biochemicals have been implicated in other stress-related phenomena and are plausible candidates for mechanisms underlying LVD in response to mental stress.
Keywords: Left ventricular dysfunction, Mental stress, Metabolomics
1 Introduction
It is commonly recognized that a number of psychosocial factors are associated with poor prognosis in patients with established coronary disease (CHD) (Hemingway and Marmot 1999; Lett et al. 2004; Rozanski et al. 1999). Transient myocardial ischemia in response to mental or emotional stress may be an important mechanistic link between psychosocial stress and adverse cardiovascular outcomes (Strike and Steptoe 2003). Mental stress induced myocardial ischemia is common in patients with CHD with prevalence estimates from studies mostly incorporating small sample sizes ranging from 20 to 70 % (Strike and Steptoe 2003). In a recent study of 310 CHD patients who did not have to have a positive exercise test for enrollment, mental stress induced ischemia was more common than exercise induced ischemia (43.5. vs. 33.8 %) (Jiang et al. 2013). One criteria for defining mental stress induced myocardial ischemia is a significant reduction of left ventricular ejection fraction (LVEF) (≥5 and ≥8) caused by mental stress test. Such stress induced LVEF reductions (i.e. left ventricular dysfunction or LVD) are believed to reflect the microvascular dysfunction of coronary vessels and have been shown to be associated with a greater risk of adverse events in CHD patients (Jiang et al. 1996; Krantz et al. 1999; Sheps et al. 2002; Babyak et al. 2010), independent of established risk indicators.
Given the prognostic importance of mental stress-induced LVD, understanding its biochemical underpinnings is an important focus of research. Studies that have investigated mediators of adverse LVEF responses in response to mental stress have been few and have suggested that catecholamines (Kuroda et al. 2000) and processes regulating increases in systemic peripheral resistance (Goldberg et al. 1996) may play roles. Those studies, however, focused on one or two variables, an approach that does not take into consideration that the underlying mechanisms whereby mental stress contributes to myocardial ischemia are complex, involving multiple biochemical pathways. For example, studies that have investigated mechanisms of emotional stress-induced blood pressure responses have suggested involvement of multiple influences exerting inhibitory and excitatory influences in the central nervous system (CNS) and in the peripheral sites. Those studies have demonstrated the importance of several tryptophan pathway compounds, such as serotonin (McCall and Clement 1994; Watts et al. 2012; Ramage and Villalón 2008), kynurenic acid (Stone 2001; Soltis and DiMicco 1992), and melatonin (Reiter et al. 2005; Tanu et al. 2007; Simko and Paulis 2007) in the regulation of emotional stress-induced cardiovascular changes. Related research points to the importance of tyrosine pathway metabolites, such as dopamine (Lokhandwala and Barrett 1982) and norepinephrine (Schroeder and Jordan 2012), and purine pathway metabolites (Kanellis and Kang 2005) in the regulation of blood pressure. Such findings suggest the importance of taking a broader approach when characterizing the biochemical underpinnings of the LVEF responses, at least in initial studies in this area.
The study of biochemistry at the global or metabolomics level can contribute significantly to our understanding of mental stress induced LVD. In contrast to traditional biochemical approaches to understanding disease that have often focused on single metabolites or single metabolic reactions, the application of metabolomics technology enables the study of multiple biochemical pathways and has been successfully applied to the study of several diseases including depression (Paige et al. 2007; Steffens et al. 2010; Ji et al. 2011), schizophrenia (Yao et al. 2010), cardiovascular disease (Shah et al. 2012; (Wang et al. 2011a), and diabetes (Wang et al. 2011b). In addition to providing a snapshot of an individual's metabolic status at a single time point, metabolomics technology can also be used to capture the flux in the biochemical pathways in response to perturbations, such as those caused by acute mental stress. Identifying biochemical changes that co-occur with LVD may provide insights into its underlying mechanisms.
In this pilot study we tested the feasibility of applying metabolomics technology to the study of the biochemical underpinnings of LVD using an electrochemistry based metabolomics (LCECA) platform. This platform allowed for the quantification compounds primarily from the tryptophan, tyrosine, and purine pathways; i.e. areas of biochemistry that have been previously shown to play a role in the CNS regulation of stress-induced cardiovascular responses. The goals of this study were threefold: (1) to examine the effects of mental stress on the levels of compounds quantitated by the LCECA, (2) examine whether pre-stress levels of compounds differ between patients with and patients without LVD, (3) examine whether stress induced changes in compounds differ in those patients with LVD.
2 Materials and Methods
2.1 Participants
Participants were male and female adult CHD patients, age 21 year or older, who were recruited for mental stress-induced myocardial ischemia screening for the REMIT (Responses of Myocardial Ischemia to Escitalopram Treatment) trial (NCT00574847) (Jiang et al. 2012). The full inclusion and exclusion criteria of the study have been previously reported (Jiang et al. 2012). This metabolomics pilot study consisted of 30 subjects who were randomly selected from the REMIT study population based on the following criteria: significant LVEF reduction ≥5 from resting to all three mental tasks, i.e. the LVD group (N = 15), and 15 who showed LVEF increases ≥5 from resting in response to all tests, i.e. normal left ventricular response or NLVR (N = 15). These groups are representative of the upper (i.e. NLVR) and lower (i.e. LVD) 15 % of the distribution of mental stress-induced LVEF responses in the REMIT study.
The study protocol was developed in accordance with the principles of the Declaration of Helsinki. The protocol was reviewed and approved by the Duke University Health System Institutional Review Board and all participants provided written, voluntary, informed consent before participating in any further assessment.
2.2 Mental stress testing
A complete description of the study procedures has been detailed previously (Jiang et al. 2012). Briefly, study subjects underwent two sessions of assessment and testing. On the first day, self-reported demographic, clinical, and psychosocial data were collected. Participants completed the stress testing protocols on the second day between 8 and 11 am at the Duke Cardiac Diagnostic Unit. Prior to stress testing, beta-blockers were withheld upon agreement of patients' cardiologist for 24–48 h, depending on the half-life of the specific medication. Upon arrival, a 19-gauge butterfly needle was inserted in the antecubital vein of the right arm to allow for acquisition of blood samples. Subjects then rested quietly for the next 20 min. to allow hormone levels to return to normal following venipuncture. Following the rest period, participants underwent three mental stress tasks in sequence: Mental arithmetic, Mirror trace, and Anger recall. A rest period of 6 min followed every stress test. Patients completed the mental stress protocol while in a left lateral position to allow for echocardiography, which was conducted during the final 3 min of each rest period and during each mental stress task. Blood samples were collected during rest prior to mental stress testing and at the end of mental stress testing approximately 25 min after the initiation of the first mental stress using a Vacutainer system containing clot activator and gel serum separator. The blood samples were centrifuged within an hour and serum was extracted and stored at −25 °C. task. LVEF was calculated by measuring the echocardiographic images of the 2 apical windows (parasternal long-axis, apical 4-chamber, and apical 2-chamber) from a 3- to 5-beat loop via the biplane Simpson's method. The American Society of Echocardiography 16-segment model was used to assess left ventricular wall motion. Each segment was graded and scored as normal (1 = normal or hyperdynamic, score) or abnormal (2 = hypokinetic, 3 = akinetic, 4 = dyskinetic, or 5 = aneurysmal) wall motion. A wall motion score index (WMSI) was calculated as the sum of the segmental wall motion scores divided by the total number of the scored segments. Separate scores were calculated for the wall motion data collected at rest, during each of the three mental stress tests. The Kappa value for the intra/inter variability of wall motion analysis in this study ranges between 0.80 and 0.87.
2.3 Metabolomic profiling
Serum samples were shipped in dry ice to a metabolomics laboratory in the Bedford Research Corporation and analyzed using an LCECA platform (Matson et al. 1984; Kristal et al. 2002). This platform is excellent for quantitating compounds that will undergo electrochemical oxidation or reduction, and includes multiple compounds from the tyrosine, tryptophan, sulfur amino acid and purine pathways, and markers of oxidative stress and protection. At the time of sample preparation, a pool was created from equal amounts of small aliquots of each sample in the study which was treated identically to a sample. This method has been extensively used and validated in prior studies in neurodegenerative and psychiatric disorders (Bogdanov et al. 2008; Rozen et al. 2005). First, all responses matching the retention and EC signature of compounds in the reference standard were exported in concentration units of ng/ml. Second, all responses matching resolved peaks in the pool of all samples are exported in terms of their relative response to the pool value. The concentrations of these are subsequently estimated by the total coulombs in the peak assuming a molecular weight of 200 and a 2 electron charge transfer. This platform yielded data for 22 known compounds, primarily within tyrosine, tryptophan, purine and methionine pathways. All personnel involved with the metabolomic profiling were blind to the clinical status of the patients and to the time of sample collection (pre-stress vs. post-stress).
2.4 Statistical methods
Descriptive statistics were computed for each group and groups were compared using Chi square analysis for categorical variables and t-tests for continuous variables. As most compounds were not normally distributed, we elected to use nonparametric techniques for the primary data analysis. The signed rank test was used to test whether compound changed significantly from pre- to post-stress. Kruskal–Wallis (KW) tests were used to test for differences between the LVD and NLVR groups. When ties were present, the normal approximations for the above KW test statistics were used in the tests. The first set of analyses focused on determining whether any pre-stress levels of compounds differed between the LVD and NLVR groups. A second set of analyses compared pre- to post-stress compound change scores between the two groups. We used percent change scores for this analysis, which were calculated by subtracting the baseline level of each compound from the corresponding post-stress level of each compound and dividing by the pre-stress values. Change was modeled in this fashion as to control for group differences in pre-stress levels of compounds. A significant KW test in this set of analyses indicates that the magnitude of the stress-induced changes in a particular compound differed between the two groups.
Control of Type I error for the first set of analyses (i.e. Pre- to post-stress change) was effected by applying the Bonferroni correction to alpha = .05 separately for each pathway (i.e. tyrosine, tryptophan, purine, one carbon metabolism, and tocopherols), a method used in previous studies utilizing this platform (Kaddurah-Daouk et al. 2012). This correction resulted in a critical alpha value of .008 (.05/6) for tyrosine metabolites, .005 (.05/10) for tryptophan metabolites, .017 (.05/3) for purine metabolites, .025 (.05/2) for the tocopherols, and .05 (.05/1) for methionine. For the two other sets of analysis, univariate tests will be followed by principal component analysis (PCA) with varimax rotation of the ranked scores (i.e. pre-stress values or stress-induced percent change scores). The purpose of the PCA was to explore whether the group differences observed in the univariate analysis might be better explained by a smaller number of components or factors. Studies have shown that this technique can be effective in identifying reliable solutions in small samples if factors are well defined and their number is limited (de Winter et al. 2009). Therefore, in order to extract those factors that are most likely to replicate in the population we examined the scree plot and retained all factors preceding the first major drop in the magnitude of the eigenvalues.
3 Results
Baseline demographic and clinical characteristics of the study groups are summarized in Table 1. The LVD and NLVR groups were similar with respect to age (p = .33), gender (p = .33) and race (p = .33). The LVD and NLVR groups were also similar with respect to resting LVEF (p = .69) and resting WMSI. However, the two groups showed very different left ventricular responses to the mental stress protocol. A comparison of the LVEF responses to stress showed a significant difference with the LVD group showing a significant drop in LVEF in response to stress and the NLVR group showing a significant increase in LVEF in response to stress. The LVD group also showed significantly greater increases in the mental stress induced WMSI than the NLVR group.
Table 1. Demographic and clinical characteristics of LVD and NLVR groups.
| Variable | LVD group | NLVR group | p value |
|---|---|---|---|
| Gender, % female | 20 | 0 | .22 |
| Race, % white | 80 | 100 | .22 |
| Age, mean (SD) | 64.53 (10.46) | 60.8 (10.13) | .33 |
| Resting LVEF mean (SD) | 56.13 (11.69) | 54.67 (8.00) | .69 |
| ΔLVEF, mean (SD) | −8.38 (2.30) | 8.07 (2.75) | <.001 |
| Resting WMSI mean (SD) | 1.32 (0.46) | 1.11 (.20) | .12 |
| ΔWMSI mean (SD) | 0.21 (0.18) | −.014 (.096) | <.001 |
LVEF left ventricular ejection fraction, WMSI wall motion score index, ΔLVEF average LVEF response to the three mental stress tasks, ΔWMSI average WMSI response to the three mental stress tasks
Results of signed rank tests on change scores of metabolites revealed several significant stress related changes in metabolite levels (Table 2). There were significant decreases in several tryptophan pathway metabolites (Idole-3-lactic acid, p < .001; 3-hydroxyky-nurenine p < .001; tryptophan, p = .003; kynurenine, p = .003; and N-acetyl serotonin, p = .022) and tyrosine pathway metabolites (tyrosine, p < .001; homogentisic acid, p < .001; and homovanillic acid, p = .014). Among the purine pathway metabolites, uric acid (p = .053) significantly decreased and hypoxanthine (p = .009) significantly increased in response to stress. Mental stress also resulted in a reduction of the sulphur amino acid, methionine (p < .001). After Bonferroni adjustment, only the changes in tyrosine, homogentisic acid, indole-3-lactic acid, 3-hydroxykynurenine, tryptophan, kynurenine, hypoxanthine, and methionine were significant.
Table 2. Stress-induced % change in compounds.
| Compound name and pathway | Pre-stress: median (frstqrt, thrdqrt) | Post-stress median (frstqrt, thrdqrt) | % change: median (frstqrt, thrdqrt) | p value |
|---|---|---|---|---|
| Tyrosine | ||||
| Tyrosine | 93.84 (75.08, 119.77) | 84.57 (71.43, 108.15) | −11.71 (−15.60, −6.21) | <.001 |
| 4-Hydroxyphenyllactic acid | 88.62 (75.60, 107.89) | 83.58 (70.02, 112.59) | −3.46 (−6.3, 4.61) | 0.19 |
| 4-Hydroxybenzoic acid | 53.80 (20.41, 130.28) | 53.21 (18.3, 133.76) | −2.72 (−10, 5.49) | 0.27 |
| Homogentisic acid | 67.88 (55.90, 106.78) | 61.04 (44.72, 91.53) | −11.05 (−27.42, −1.23) | <.001 |
| Methoxy-hydroxyphenly glycol | 87.71 (75.10, 111.06) | 95.89 (77.16, 109.87) | .65 (−5.77, 7.51) | 0.54 |
| Homovanillic acid | 80.34 (66.71, 104.77) | 73.49 (54.85, 92.10) | −9.40 (−21.03, −1.87) | 0.014 |
| Tryptophan | ||||
| Tryptophan | 99.46 (85.02, 105.25) | 94.72 (80.14, 99.52) | −3.66 (−7.66, .01) | 0.003 |
| 5-hydroxytryptophan | 1.1 (.1, 2.16) | 1.18 (.1, 1.94) | 0 (−12.61, 3.73) | 0.68 |
| Serotonin | 76.85 (36.46, 122.42) | 85.31 (47.47, 135.1) | 8.79 (−22.65, 62.49) | 0.14 |
| 5-Hydroxyindoleacetic acid | 75.17 (56.56, 110.81) | 75.14 (51.02, 101.18) | −5.63 (−18.66, 6.03) | 0.27 |
| N-acetylserotonin | 81.56 (63.49, 111.94) | 76.99 (66.94, 103.72) | −4.6 (−7.67, .17) | 0.022 |
| Kynurenine | 98.24 (87.9, 111.78) | 93.60 (83.37, 109.83) | −3.92 (−8.86, .23) | 0.003 |
| 3-Hydroxykynurenine | 90.04 (71.40, 118.61) | 79.92 (62.76, 100.64) | −9.2 (−13.34, −1.59) | <.001 |
| 3-Hydroxyanthranillic acid | 4.48 (.10, 53.71) | 3.13 (.10, 45.81) | 0 (−14.71, 6.42) | 0.84 |
| Indole-3-lactic acid | 85.50 (65.22, 115.03) | 76.24 (59.43, 108.56) | −8.54 (−13.37, −5.48) | <.001 |
| Indole-3-propionic acid | 68.68 (41.64, 125.43) | 64.27 (42.58, 92.46) | −3.50 (−9.98, 3.60) | 0.14 |
| Purine | ||||
| Hypoxanthine | 71.41 (59.75, 88.94) | 87.17 (73.49, 106.56) | 16.34 (−2.0, 33.26) | 0.009 |
| Xanthine | 83.54 (70.68, 108.52) | 87.64 (68.61, 110.82) | −1.31 (−6.14, 13.51) | 0.88 |
| Uric Acid | 94.80 (90.10, 102.64) | 93.96 (87.01, 103.10) | −.86 (−2.89, .84) | 0.053 |
| One-carbon metabolism | ||||
| Methionine | 97.3 (77.7, 111.96) | 86.23 (71.27, 100.12) | −10.54 (−17.60, −5.31) | <.001 |
| Tocopherol | ||||
| Tocopherol-gamma | 45.73 (31.95, 98.20) | 42.62 (25.95, 83.52) | −9.81 (−35.19, 5.38) | 0.17 |
| Tocopherol-alpha | 72.52 (47.46, 80.57) | 64.86 (38.70, 83.69) | −11.47 (−33.51, 12.54) | 0.23 |
A comparison of pre-stress compound levels across the two groups revealed several significant differences between the LVD and the NLVR groups (see Table 3). The LVD group showed higher pre-stress levels of alpha-tocopherol (p = .014), gamma tochopherol (p = .040) and, lower levels of 3-hydroxykynurenine (3-OHKY, p = .033) and 4-hydroxyphenyllactic acid (p = .036). There was also a trend for the LVD group to have lower levels of N-acetyl serotonin (NA5HT) compared to the NLVR group (p = .089). PCA of the pre-stress metabolites revealed 7 components with an eigenvalue above 1. The eigenvalues for the first components were 4.62, 3.56, and 2.05 respectively. Examination of the scree plot resulted in us retaining the first component, which accounted for 21.04 % of the variance. This component was primarily defined by very strong loadings (≥.70) for homogentisic acid, and the amino acids tyrosine, tryptophan, and methionine and less strong, but still significant loadings (>.5 and <.70) from N-actylserotonin, 4-Hydroxyphenyllactic acid, and Indole-3-lactic acid. A comparison of the component scores between the LVD and NLVR groups, however, revealed a nonsignificant effect (p = .13).
Table 3. Comparisons of pre-stress compound levels between LVD and NLVR groups.
| Variable | Pathway | p value | LVD Median (frstqrt, thrdqrt) | NLVR Median (frstqrt, thrdqrt) |
|---|---|---|---|---|
| Methoxy-hydroxyphenly glycol | TYR | 0.40 | 86.13 (70.71, 111.06) | 98.17 (79.41, 111.51) |
| Homogentisic acid | TYR | 0.42 | 65.55 (36.49, 106.78) | 69.06 (58.26, 142.48) |
| Tyrosine | TYR | 0.48 | 84.74 (70.19, 120.45) | 102.24 (75.51, 119.52) |
| 4-Hydroxyphenyllactic acid | TYR | 0.036 | 83.69 (61.95, 101.80) | 94.54 (85.01, 125.94) |
| 4-Hydroxybenzoic acid | TYR | 0.55 | 40.06 (9.95, 146.62) | 85.11 (23.85, 130.28) |
| Homovanillic acid | TYR | 0.58 | 81.10 (66.71, 107.29) | 73.17 (56.30, 104.77) |
| 3-Hydroxykynurenine | TRP | 0.033 | 76.46 (49.30, 91.79) | 113.30 (79.58, 143.58) |
| N-acetylserotonin | TRP | 0.089 | 73.79 (62.51, 91.31) | 90.37 (78.58, 127.71) |
| Indole-3-propionic acid | TRP | 0.29 | 68.35 (28.11, 83.89) | 83.03 (41.64, 208.38) |
| 3-Hydroxyanthranillic acid | TRP | 0.32 | 2.97 (0.10, 53.71) | 8.08 (0.10, 59.73) |
| Tryptophan | TRP | 0.44 | 93.54 (85.45, 102.74) | 101.52 (77.12, 109.80) |
| Indole-3-lactic acid | TRP | 0.47 | 76.45 (63.27, 115.03) | 89.75 (65.66, 120.44) |
| 5-Hydroxyindoleacetic acid | TRP | 0.47 | 71.54 (47.23, 107.60) | 78.80 (57.13, 114.19) |
| 5-hydroxytryptophan | TRP | 0.63 | 1.12 (0.10, 2.16) | 1.07 (0.10, 2.17) |
| Kynurenine | TRP | 0.63 | 98.17 (89.56, 110.17) | 100.27 (82.27, 119.22) |
| Serotonin | TRP | 0.92 | 79.25 (36.46, 113.71) | 74.46 (33.13, 125.41) |
| Xanthine | PUR | 0.21 | 91.54 (71.10, 119.54) | 78.64 (69.74, 99.44) |
| Uric Acid | PUR | 0.95 | 96.50 (87.83, 102.64) | 93.65 (90.10, 104.11) |
| Hypoxanthine | PUR | 0.98 | 70.85 (59.75, 89.01) | 80.44 (55.43, 88.94) |
| Methionine | OCM | 0.37 | 91.79 (71.75, 110.43) | 103.60 (80.28, 133.27) |
| Tocopherol-alpha | TOC | 0.014 | 78.39 (73.45, 86.25) | 58.59 (33.22, 69.64) |
| Tocopherol-gamma | TOC | 0.040 | 85.52 (34.69, 112.42) | 37.31 (22.46, 57.01) |
frstqrt first quartile, thrdqrt third quartile, TYR tyrosine, TRP tryptophan, PUR purine, OCM one carbon metabolism, TOC tocopherol
A comparison of the mental stress induced change scores between the LVD and NLVR groups revealed significant effects for kynurenine (p = .036), uric acid (p = .015), N-acetylserotonin (p = .054), and tyrosine (p = .019) indicating that the LVD and NLVR groups responded differently to the mental stress protocol (Table 4; Fig. 1a–d). In general, mental stress resulted in reductions in these compounds, but the magnitude of those reductions was greater for the NLVR group. The stress-related methionine reduction was also greater in NLVR group, though statistically it was not significant (p = .065, Fig. 1e). PCA of the change scores revealed 9 components with an eigenvalue above 1. The eigenvalues for the first three components were 4.98, 2.81, and 2.25, respectively. Examination of the scree plot resulted in us retaining the first component which accounted for 22.64 % of the variance of the change scores. The variables that had significant loadings (>.5) on this component were: uric acid, kynurenine, tyrosine, methionine, Indole-3-lactic acid, 4-hydroxyphenyllactic acid, indole-3-propionic acid, 3-hydroxykynurenine, and tryptophan. Thus, many of the compounds showing significant pre- to post stress reductions were strongly correlated with this component. This suggests that individuals showing a relatively large reduction in one of those compounds are tending to show a large reduction in the other compounds. A KW test comparing the component score between the LVD and NLVR groups was significant (p = .019) with the NLVDR group showing lower scores than the LVD group. This suggests that the differences in change scores between the NLVR and LVD groups reported above likely reflect a more general tendency for the NLVR group to respond to mental stress with a greater reduction in the compounds that showed significant decreases from pre- to post-stress.
Table 4. Comparisons of stress-induced % changes between LVD and NLVR groups.
| Compound name and Pathway | LVD pre-stress: median (frstqrt, thrdqrt) | LVD post-stress: median (frstqrt, thrdqrt) | NLVR pre-stress: median (frstqrt, thrdqrt) | NLVR post-stress: median (frstqrt, thrdqrt) | LVD % change: median (frstqrt, thrdqrt) | NLVR % change: median (frstqrt, thrdqrt) | p-value |
|---|---|---|---|---|---|---|---|
| Tyrosine | |||||||
| Tyrosine | 84.74 (70.19, 120.45) | 79.26 (63.65, 116.05) | 102.24(75.51, 119.52) | 88.27 (73.84, 100.00) | −7.68 (−11.72, −1.33) | −14.97 (−16.33, −11.71) | 0.019 |
| 4-Hydroxyphenyllactic acid | 83.69 (61.95, 101.08) | 72.43 (64.31, 97.72) | 94.54 (85.01, 125.94) | 97.20 (82.59, 122.62) | −3.77 (−8.33, 4.61) | −3.16 (−6.30, 4.98) | 0.98 |
| 4-Hydroxybenzoic acid | 40.06 (9.95, 146.62) | 38.09 (10.13, 165.02) | 85.11 (23.85, 130.28) | 73.40 (18.37, 130.21) | −1.04 (−5.88, 5.49) | −2.80 (−10.52, 8.69) | 0.55 |
| Homogentisic acid | 65.55 (36.49, 106.78) | 64.70 (33.98, 90.91) | 69.06 (58.26, 142.48) | 60.06 (48.41, 95.34) | −8.16 (−18.46, 2.54) | −13.03 (−31.06, −8.07) | 0.18 |
| Methoxy-hydroxyphenly glycol | 86.13 (70.71, 111.06) | 85.39 (69.10, 109.17) | 98.17 (79.41, 111.51) | 100.61 (80.74, 112.65) | −.86 (−6.11, 12.76) | .88 (−5.50, 5.69) | 0.66 |
| Homovanillic acid | 81.10 (66.71, 107.29) | 87.47 (56.11, 122.33) | 73.17 (56.30, 104.77) | 71.80 (46.49, 90.62) | −8.19 (−12.15, 10.16) | −14.44 (−34.58, −7.82) | 0.10 |
| Tryptophan | |||||||
| Tryptophan | 93.54 (85.45, 102.74) | 95.40 (80.40, 99.50) | 101.52(77.12, 109.80) | 94.05 (77.90, 101.83) | −3.58 (−5.22, 0.89) | −4.19 (−11.05, −.03) | 0.29 |
| 5-Hydroxytryptophan | 1.12 (.1, 2.16) | 1.32 (.10, 1.94) | 1.07 (.10, 2.17) | 1.04 (.10, 1.98) | 0 (−10.19, 9.43) | 0 (−17.20, 3.73) | 0.88 |
| Serotonin | 79.25 (36.46, 113.71) | 82.71 (42.76, 117.70) | 74.46 (33.13, 125.41) | 87.91 (49.54, 138.47) | −.23 (−26.15, 59.87) | 10.39 (−22.65, 84.91) | 0.55 |
| 5-Hydroxyindoleacetic acid | 71.54 (47.23, 107.60) | 71.58 (49.67, 111.80) | 78.80 (57.13, 114.19) | 84.87 (63.51, 101.18) | −.97 (−19.17, 13.77) | −9.72 (−18.66, 6.03) | 0.49 |
| N-Acetylserotonin | 73.79 (62.51, 91.31) | 72.53 (60.61, 95.41) | 90.37 (78.58, 127.71) | 85.02 (74.31, 113.81) | −4.10 (−5.70, 5.50) | −6.83 (−12.58, −1.19) | 0.054 |
| Kynurenine | 98.17 (89.56, 110.16) | 92.78 (83.37, 109.83) | 100.27 (82.27, 119.22) | 94.43 (74.98, 112.77) | −3.20 (−6.05, 3.28) | −8.86 (−9.84, −1.34) | 0.036 |
| 3-Hydroxykynurenine | 76.46 (49.3, 91.79) | 67.18 (47.65, 86.83) | 113.30(79.58, 143.58) | 93.10 (68.73, 116.73) | −7.22 (−12.19, 0) | −10.29 (−22.05, −1.59) | 0.33 |
| 3-Hydroxyanthranillic acid | 2.97 (.10, 53.71) | 2.01 (.10, 45.81) | 8.08 (.10, 59.73) | 7.91 (.10, 51.30) | 0 (−9.34, 16.16) | −12.65 (−30.96, 6.42) | 0.33 |
| Indole-3-lactic acid | 76.45 (63.27, 115.03) | 69.89 (58.71, 108.56) | 89.75 (65.66, 120.44) | 79.71 (61.55, 113.56) | −7.38 (−12.63, −1.71) | −10.02 (−17.01, −5.71) | 0.15 |
| Indole-3-propionic acid | 68.35 (28.11, 83.89) | 62.32 (42.01, 80.48) | 83.03 (41.64, 208.38) | 68.12 (42.88, 185.63) | −1.07 (−8.75, 19.35) | −3.60 (−11, 2.39) | 0.60 |
| Purine | |||||||
| Hypoxanthine | 70.85 (59.75, 89.01) | 86.17 (55.21, 103.74) | 80.44 (55.43, 88.94) | 94.08 (73.49, 111.91) | −.86 (−20.76, 47.74) | 17.32 (8.29, 33.26) | 0.35 |
| Xanthine | 91.54 (71.10, 119.54) | 90.81 (70.18, 117.18) | 78.64 (69.74, 99.44) | 80.37 (67.05, 93.48) | −1.34 (−12.29, 11.33) | 1.17 (−5.43, 13.53) | 0.44 |
| Uric acid | 96.50 (87.83, 102.64) | 96.20 (86.32, 103.92) | 93.65 (90.10, 104.11) | 89.70 (87.01, 103.10) | .54 (−1.72, 1.35) | −2.42 (−6.93, −.54) | 0.015 |
| One-carbon metabolism | |||||||
| Methionine | 91.79 (71.75, 110.43) | 86.92 (71.26, 99.41) | 103.06 (80.28, 133.27) | 85.54 (71.13, 110.84) | −8.71 (−16.28, −2.77) | −13.83 (−23.00, −10.25) | 0.065 |
| Tocopherol | |||||||
| Tocopherol-gamma | 85.52 (34.68, 112.42) | 81.72 (27.76, 109.30) | 37.31 (22.46, 57.01) | 34.26 (17.29, 48.77) | −13.61 (−27.31,4.37) | −6.01 (−41.30, 17.19) | 0.73 |
| Tocopherol-alpha | 78.39 (73.45, 86.25) | 67.95 (39.86, 83.69) | 58.59 (33.22, 69.64) | 48.37 (25.75, 99.25) | −16.34 (−30.24, 10.46) | 3.40 (−35.13, 21.96) | 0.44 |
Fig. 1.
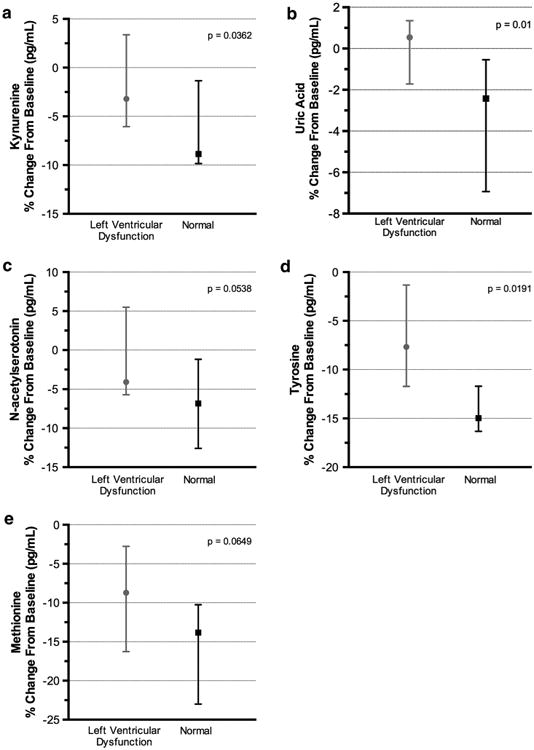
(a) Stress-induced change in kynurenine in NLVR and LVD groups, (b) Stress-induced change in uric acid in NLVR and LVD groups, (c) Stress-induced change in N-acetyl serotonin in NLVR and LVD groups (d) Stress-induced change in tyrosine in NLVR and LVD groups, and (e) Stress-induced change in methionine in NLVR and LVD groups
4 Discussion
This pilot study identified biochemical compounds in several pathways that were significantly different between patients with LVD and patients with a normal left ventricular response to mental stress. To our knowledge, this the first study in humans to have investigated the effects of mental stress on our selected areas of biochemistry primarily compounds of the tryptophan, tyrosine, and purine pathways. Overall, mental stress appeared to exert broad effects on these compounds, resulting in significant changes in 11 of the 22 compounds that were investigated. Given the importance of these pathways to physiological function, such changes may have relevance for understanding stress-disease relationships. Comparisons between the LVD and NLVR groups demonstrated a number of differences in the investigated pathways. Many of these differences appear to be plausibly linked to cardiovascular function and may provide important insight into understanding LVD in response to mental stress.
Among the various changes that occurred was a significant stress-induced increase in the purine compound, hypoxanthine. This may reflect mitochondrial dysfunction due to oxidative stress, a condition that is believed to play a role in the etiology of several diseases (Warda et al. 2013). Mitochondria convert adenosine diphosphate (ADP) to adenosine-5′-triphosphate (ATP) which is used as a source of cellular energy. During periods of oxidative stress mitochondria become less efficient in converting ADP to ATP resulting in ADP being shunted to the production of hypoxanthine resulting in an increase in that compound, as seen in this study. There was also a general trend for a reduction in compounds with anti-oxidant properties, such as the tryptophan compound, indole-3-propionic acid (Bendheim et al. 2002) and the tocopherols. These reductions may reflect the utilization of these compounds to limit the damage caused by oxidative stress.
Mental stress also resulted in alterations in the tryptophan, tyrosine, and methionine pathways. Some of the strongest effects were significant reductions in the amino acids tyrosine, methionine, and to a lesser extent tryptophan. A reduction in amino acids in response to acute (Teague et al. 2007) and chronic (Xiaoxia Gaoa et al. 2011; Maren Depke et al. 2008) stress has been reported in previous studies of rats and most likely indicates an increase in the utilization of these compounds in synthesis of pathway products. Although multiple compounds in the tryptophan and tyrosine pathways showed significant stress-induced changes, the effects were not uniform across all branches. Of the nine tryptophan pathway compounds quantitated by the LCECA platform, five showed significant decreases in response to stress. The tryptophan pathway contains two major branches, i.e. kynurenine and serotonin (Fig. 2). The significant decreases in tryptophan, kynurenine, and 3-hydroxykynurenine and non-significant changes in 5-hydroxytryptophan (i.e. the precursor to serotonin), serotonin, and 5-hydroxyindoleactic acid, the major metabolite of serotonin suggest that mental stress resulted in a greater alteration of the kynurenine branch of tryptophan metabolism. Levels of N-acetyl serotonin decreased and its precursor 5HT, which in serum is primarily derived from platelet uptake from the enterochromaffin cells in the gut, showed a slight, but non-significant increase. This suggests a possible impact of the stressor on the activity of N acetyl transferase, the enzyme that converts serotonin to N-acetyl serotonin. Indole-3-propionic acid, a product of the clostridium sporogines in the gut microbiome, which acts as both an antioxidant and in the prevention of protein aggregation (Bendheim et al. 2002) also declined in response to mental stress. We speculate that this decrease in Indole-3-propionic acid reflects its utilization as an anti-oxidant secondary to mental stress-induced increase in free radical production. The other possibility is that the decrease in Indole-3-propionic acid is the result of a direct effect on gut production of this metabolite over the course of the mental stress protocol. If this is the case it would have to be specific change in the production of Indole-3-propionic acid by the gut micro flora rather than a general effect on the gut given that there was minimal change in pre- to post-stress levels of serotonin which is produced primarily by the enterochromaffin cells in the gut.
Fig. 2.
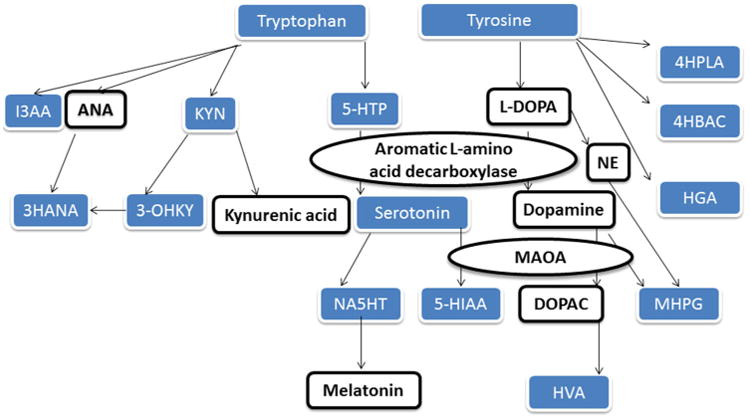
Tryptophan and Tyrosine pathway metabolites. Blue shaded boxes are compounds quantitated by the LCECA platform in this study. I3AA indole-3-lactic acid, 3-OHAN 3-hydroxyanthranillic acid, KYN kynurenine, 3-OHKY 3-Hydroxykynurenine, 5-HTP 5-hydroxytryptophan, NA5HT N-acetyl serotonin, 5-HIAA 5-Hydroxyindoleacetic acid, HVA homovanillic acid, 4HPLA 4-hydroxyphenyllactic acid, 4HBAC 4-hydroxybenzoic acid, HGA homogentisic acid, MHPG methoxy-hydroxyphenly glycol, ANA anthranilic acid, NE norepinephrine, L-DOPA levo dopa, DOPAC 3,4-Dihydroxyphenylacetic acid, MAOA Monoamine oxidase A
Mental stress also appeared to have differential effects on branches of tyrosine metabolism. The significant decreases in tyrosine and homovanillic acid, the major metabolite of dopamine, and the non-significant change in methoxy-hydroxyphenly glycol, the major metabolite of norepinephrine, indicate greater alteration in dopaminergic than noradrenergic function. This would suggest that the mental stress protocol did not evoke an adrenal medulla mediated elevation in norepinephrine. The significant changes in tyrosine and homovanillic acid may reflect a stress-induced increase in utilization of tyrosine with a subsequent decrease in homovanaillic acid due to the reduction of its precursor (i.e. tyrosine). Alternatively the mental stress protocol might have induced lower activity in the catechol-O-methyltransferase, an enzyme that plays a major role in dopamine degradation, resulting in decreases in the homovanillic acid.
The findings that mental stress resulted in alterations of the kynurenine pathway are particularly interesting because some of those compounds appear to be important for distinguishing between LVD and NLVR groups. Pre-stress levels of 3-hydroxykynurenine acid were lower in LVD group compared to the NLVR group. Mental stress caused a significantly greater reduction in kynurenine, the precursor to kynurenic acid, in the NLVR group than in the LVD group. The involvement of the kynurenine pathway in LVD seems plausible as previous research has suggested that kynurenic acid exerts an inhibitory effect on cardiovascular activity via antagonizing excitatory glutamate receptors in the CNS (Stone 2001; Soltis and DiMicco 1992) and at peripheral sties via acting as endothelium relaxation agent (Wang et al. 2010). Though speculative, the greater decreases in kynurenine seen among those patients in the NLVR group may reflect a greater utilization of this compound to synthesize cardio-inhibitory compound kynurenic acid.
The N-acetyl serotonin findings are also intriguing. N-acetyl serotonin is the precursor to melatonin, a compound that has been shown to have cardio protective properties via action in the CNS and at peripheral sites (Reiter et al. 2005; Simko and Paulis 2007; Tanu et al. 2007; Dubocovich et al. 2010). N-acetyl serotonin can function as a melatonin agonist at certain receptors (Oxenkrug 1999), so it is possible that N-acetyl serotonin might influence stress responses via activation of melatonin receptors. N-acetyl serotonin also appears to have cardiac effects independent of melatonin (Oxenkrug 1999). Thus, the greater decreases in N-acetyl serotonin seen among those patients in the NLVR group may reflect greater uptake or utilization of these compounds that have been reported to have inhibitory effects on cardiovascular function.
The greater reduction in tyrosine and the trend for a greater reduction in the dopamine metabolite homovanillic acid seen among the patients in the NLVR group may indicate a possible role for dopamine in LVD. Previous reports indicate that dopamine has an inhibitory effect on cardiovascular activity possibly mediated through inhibition of norepinephrine release via activation of the D2 receptors (Yoon et al. 1994; Végh et al. 1998). One recent study (Végh et al. 1998) of canines administration of the dopamine agonist Z1046 resulted in reduced severity of ischemia (including ST-segment elevation and ventricular ectopic activity) induced by a 25-min occlusion of the left anterior descending coronary artery. Concomitant reductions in arterial blood pressure and heart rate suggest that the anti-ischemic effects of Z1046 were due to inhibition of cardiac sympathetic responses. We did not have direct measure of dopamine in our study, but these results suggest that its measurement may be important future studies examining biochemical mediators of mental stress-induced LVD.
Another noteworthy finding included a significant reduction in uric acid, with greater reductions being observed in the NLVR group. Uric acid is believed to be a contributor to endothelial dysfunction (Kanellis and Kang 2005. Uric acid is also a potent antioxidant in the CNS and is believed to play a protective role in a host of CNS disease due to its neuroprotective properties (Kutzing and Firestein 2008). Methionine levels also showed a stark decrease in response to stress and this reduction tended to be greater in the NLVR group suggesting a role for the methionine cycle in stress-induced LVD. Methionine and its derivative, S-adenosyl methinione have been shown to have antidepressant effect possibly through DNA methylation (Papakostas et al. 2003; Cantoni et al. 1989; Kagan et al. 1990). The potential role of methionine and related compounds to depression may hold relevance to LVEF responses, as depressive symptoms have been shown to be associated with an abnormal mental stress induced LVEF response (Jiang et al. 2003; Boyle et al. 2013).
Using principal components analysis on the stress-induced change scores we identified one component with significant loadings from many of the metabolites that showed significant pre- to post-stress changes. Among the highest loading change scores were the amino acids tyrosine, tryptophan, and methionine. The NLVR group had significantly lower component scores indicating that this group showed a general tendency to react to mental stress with a greater reduction in compounds measured by the LCECA. At this time, we can only speculate about the mechanism that accounts for the correlated change in these compounds. One possibility is that the reduction in many of these compounds reflects increased uptake into the CNS for utilization in the production of various compounds that are utilized during the experience of mental stress. It is well documented that various compounds in the CNS (e.g. tryptophan products, such as kynurenic acid (Soltis and DiMicco 1992; Miura et al. 2008; Oxenkrug 2013), serotonin (McCall and Clement 1994; Ramage and Villalón 2008; Miura et al. 2008; Oxenkrug 2013), N-acetyl-sero-tonin (Oxenkrug 1999; Oxenkrug and Ratner 2012), and melatonin (Simko and Paulis 2007; Catena-Dell'Osso et al. 2012) play roles in modulating physiological and psychological responses during stress. Synthesis of these compounds, which is essential for maintaining healthy neurotransmission, is dependent on the transport of precursor amino acids across the blood brain barrier. Thus, the observation that patients showing adverse cardiovascular responses to mental stress (i.e. LVD patients) showed less reduction in these compounds from pre- to post-stress might suggest that they possess some impairment in their ability to transport amino acids and/or other essential compounds (i.e. kynurenine) across the blood brain barrier. Such impairments have been shown experimentally to contribute to neurobehavioral dysfunction indicative of anxiety-like behaviors in mice (Coppola et al. 2013) and could plausibly contribute to other stress-related phenomena, such as altered cardiovascular function during stress. It is relevant that indices of poor psychological functioning, such as measures of depressive symptoms and anxiety, are associated with stress-induced LVD (Boyle et al. 2013; Miura et al. 2008) and/or other pathogenic stress-induced cardiovascular responses (Edmondson et al. 2013) and that many of the CNS metabolic pathways that are believed to play roles in those psychological dimensions also appear to regulate cardiovascular responses to stress (e.g. tryptophan products, such as kynurenic acid (Soltis and DiMicco 1992), serotonin (McCall and Clement 1994; Ramage and Villalón 2008, N-acetyl-serotonin (Oxenkrug 1999), and melatonin (Simko and Paulis 2007). Though plausible, such an explanation is speculative at this time and requires further study.
This study has limitations. The LCECA platform quantitated 22 compounds resulting in a large number of statistical tests, thus increasing the chances for false positives. However, many of the compounds that were different between the two groups are also compounds that have been implicated in other stress related processes. Therefore, this increases our confidence that our findings do not simply reflect chance. The small sample size also limited the power to detect smaller, but still meaningful effect sizes, particularly for those comparisons between the LVD and NLVR groups. With these limitations in mind, the findings of this study and the speculations about mechanisms should be viewed as hypotheses to be tested in a larger cohort and with the application of targeted methods to measure more of the compounds in the pathways investigated in this study.
5 Conclusion
In summary, this small but novel study utilized metabolomics technology to begin to map the biochemical underpinnings of LVD in response to mental stress. Our data demonstrate that the LCECA platform, specifically, and that metabolomics approaches, in general, hold the potential to elucidate biochemical pathways and networks regulating mental stress induced LVD. Several areas of biochemistry that appear to be plausible candidates for being a mechanistic link between the experience of mental stress and LVD were identified in this study. Such findings allowed for speculation of the underlying metabolic processes involved in mental stress-induced LVD, thus forming the basis for hypotheses to be tested in future studies.
Acknowledgments
The authors would also like to thank Ye Zhang, MA, Kevin Prybol, MPH, and Kaitlyn Weinberg for their contributions to the creation of figures and tables for this paper and their assistance in editing the manuscript. The study was funded by National Heart, Lung, and Blood Institute, grant number R01HL085704.
Funding Source The REMIT study was funded by the National Heart Lung and Blood Institute (NHLBI, R01HL085704), Bethesda, Maryland.
Footnotes
Conflict of interest Dr. Redford B. Williams reports holding a U.S. patent on the 5HTTLPR L allele for use as a marker of increased cardiovascular risk in stressed persons and is a founder and major stockholder of Williams LifeSkills, Inc. Dr. Velazquez reports receiving research grants from Abbott Laboratories, Evalve, and Ikaria, and consulting fees from Boehringer Ingelheim, Gilead, and Novartis. Dr. O'Connor reports receiving funding from the following: Actelion Pharmaceuticals Ltd., Amgen, Inc., Biscardia, LLC, Cardiology Consulting Associates, Faculty Connection, GE Healthcare, Ikaria, Neurotronik/Interventional Autonomics Corporation, Novella Clinical, Inc., Pfizer Inc., Pozen, and Roche Diagnostics. Dr. Wayne R. Matson is currently Chief Scientist at Counterpoint Health Solutions and is involved in developing patens in disease risk factors based on metabolomics.
Contributor Information
Stephen H. Boyle, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3366, Durham, NC 27710, USA
Wayne R. Matson, Department of Systems Biochemistry, Counterpoint Health Solutions Inc, Bedford, MA, USA
Eric J. Velazquez, Department of Medicine, Duke University Medical Center, Durham, NC, USA
Zainab Samad, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Redford B. Williams, Jr, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3366, Durham, NC 27710, USA.
Swati Sharma, Department of Systems Biochemistry, Counterpoint Health Solutions Inc, Bedford, MA, USA.
Beena Thomas, Department of Systems Biochemistry, Counterpoint Health Solutions Inc, Bedford, MA, USA.
Jennifer L. Wilson, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3366, Durham, NC 27710, USA
Christopher O'Connor, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Wei Jiang, Email: jiang001@mc.duke.edu, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3366, Durham, NC 27710, USA, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
References
- Babyak MA, Blumenthal JA, Hinderliter A, et al. Prognosis after change in left ventricular ejection fraction during mental stress testing in patients with stable coronary artery disease. American Journal of Cardiology. 2010;105:25–28. doi: 10.1016/j.amjcard.2009.08.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA, Chain DG. Development of indole-3-propionic acid (OXIGON) for Alzheimer's disease. Journal of Molecular Neuroscience. 2002;19(1–2):213–217. doi: 10.1007/s12031-002-0036-0. [DOI] [PubMed] [Google Scholar]
- Bogdanov M, et al. Metabolomic profiling to develop blood biomarkers for Parkinson's disease. Brain. 2008;31:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- Boyle SH, Samad Z, Becker RC, Williams R, Kuhn C, Ortel TL, Kuchibhatla M, Prybol K, Rogers J, O'Connor C, Velazquez EJ, Jiang W. Depressive symptoms and mental stress-induced myocardial ischemia in patients with coronary heart disease. Psychosomatic Medicine. 2013;75(9):822–831. doi: 10.1097/PSY.0b013e3182a893ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni GL, Mudd SH, Andreoli V. Affective disorders and S-adenosylmethionine: A new hypothesis. Trends in Neurosciences. 1989;12:319–324. doi: 10.1016/0166-2236(89)90038-6. [DOI] [PubMed] [Google Scholar]
- Catena-Dell'Osso M, Marazziti D, Rotella F, Bellantuono C. Emerging target for the pharmacological treatment of depression: focus on melatonergic system. Current Medicinal Chemistry. 2012;19(3):428–37. doi: 10.2174/092986712803414277. [DOI] [PubMed] [Google Scholar]
- Coppola A, Wenner BR, Ilkayeva O, Stevens RD, Maggioni M, Slotkin TA, Levin ED, Newgard CB. Branched-chain amino acids alter neurobehavioral function in rats. American Journal of Physiology—Endocrinology and Metabolism. 2013;304(4):E405–13. doi: 10.1152/ajpendo.00373.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winter JFC, Dodou D, Wieringa PA. Exploratory factor analysis with small sample sizes. Multivariate Behavioral Research. 2009;44(2):147–181. doi: 10.1080/00273170902794206. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacological Reviews. 2010;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Newman JD, Whang W, Davidson KW. Emotional triggers in myocardial infarction: do they matter? European Heart Journal. 2013;34(4):300–6. doi: 10.1093/eurheartj/ehs398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94(10):2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Marmot M. Evidence based cardiology: Psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hebbring S, Zhu H, et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: Pharmacometabolomics-informed pharmacogenomics. Clinical Pharmacology and Therapeutics. 2011;89(1):97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Babyak M, Krantz DS, et al. Mental stress-induced myocardial ischemia and cardiac events. Jama-J Am Med Assoc. 1996;275:1651–1656. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- Jiang W, Babyak MA, Rozanski A, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. American Heart Journal. 2003;146:55–61. doi: 10.1016/S0002-8703(03)00152-2. [DOI] [PubMed] [Google Scholar]
- Jiang W, Velazquez EJ, Samad Z, Kuchibhatla M, Martsberger C, Rogers J, et al. Responses of mental stress-induced myocardial ischemia to escitalopram treatment: background, design, and method for the responses of mental stress induced myocardial ischemia to escitalopram treatment trial. American Heart Journal. 2012;163(1):20–26. doi: 10.1016/j.ahj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Yuan P, Boyle SH, Matson W, Wang Z, Zeng ZB, Zhu H, Dougherty GG, Yao JK, Chen G, Guitart X, Carlson PJ, Neumeister A, Zarate C, Krishnan RR, Manji HK, Drevets W. Cerebrospinal fluid metabolome in mood disorders-remission state has a unique metabolic profile. Science Report. 2012;2:1–10. doi: 10.1038/srep00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan BL, Sultzer DL, Rosenlicht N, Gerner RH. Oral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry. 1990;147:591–595. doi: 10.1176/ajp.147.5.591. [DOI] [PubMed] [Google Scholar]
- Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Seminars in Nephrology. 2005;25(1):39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Santiago HT, Kop WJ, et al. Prognostic value of mental stress testing in coronary artery disease. American Journal of Cardiology. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Vigneau-Callahan K, Matson WR. Simultaneous analysis of multiple redox-active metabolites from biological matrices. Methods in Molecular Biology. 2002;186:185–194. doi: 10.1385/1-59259-173-6:185. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Kuwabara Y, Watanabe S, et al. Effect of mental stress on left ventricular ejection fraction and its relationship to the severity of coronary artery disease. European Journal of Nuclear Medicine. 2000;27:1760–1767. doi: 10.1007/s002590000383. [DOI] [PubMed] [Google Scholar]
- Kutzing M, Firestein BL. Altered uric acid levels and disease states. Journal of Pharmacology and Experimental Therapeutics. 2008;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: Evidence, mechanisms, and treatment. Psychosomatic Medicine. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- Lokhandwala MF, Barrett RJ. Cardiovascular dopamine receptors: Physiological, pharmacological implications. Journal of Autonomic Pharmacology. 1982;2(3):189–215. doi: 10.1111/j.1474-8673.1982.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Maren Depke M, Gerhard Fusch G, Grazyna Domanska G, Robert Geffers R, Uwe VÖlker U, Christine Schuett C, Cornelia Kiank C. Hypermetabolic Syndrome as a Consequence of repeated psychological stress in mice. Endocrinology. 2008;149(6):2714–2723. doi: 10.1210/en.2008-0038. [DOI] [PubMed] [Google Scholar]
- Matson W, Langials P, Volicer L, Gamache P, Bird E, Mark K. N-electrode three dimensional liquid chromatography with electrochemical detection for determination of neurotransmitters. Clinical Chemistry. 1984;30:1477–1488. [PubMed] [Google Scholar]
- McCall RB, Clement ME. Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. Pharmacological Reviews. 1994;46:231–243. [PubMed] [Google Scholar]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11(3):198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin. A review. Journal of Neurobiology. 1999;7:213–224. [PubMed] [Google Scholar]
- Oxenkrug G, Ratner R. N-acetylserotonin and aging-associated cognitive impairment and depression. Aging & Disease. 2012;3(4):330–8. [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G. Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Current Drug Targets. 2013;14(5):514–21. doi: 10.2174/1389450111314050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige LA, Mitchell MW, Krishnan KR, et al. A preliminary metabolomic analysis of older adults with and without depression. International Journal of Geriatric Psychiatry. 2007;22:418–423. doi: 10.1002/gps.1690. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Alpert JE, Fava M. S-adenosyl-methionine in depression: A comprehensive review of the literature. Current Psychiatry Reports. 2003;5(6):460–466. doi: 10.1007/s11920-003-0085-2. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Villalón CM. 5-Hydroxytryptamine and cardiovascular regulation. Trends in Pharmacological Sciences. 2008;29(9):472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: Physiology versus pharmacology. Journal of Pineal Research. 2005;39:215–216. doi: 10.1111/j.1600-079X.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Rozen S, Cudkowicz ME, Bogdanov M, Matson WR, Kristal BS. Metabolomic analysis and signatures in motor neuron disease. Metabolomics. 2005;1:101–108. doi: 10.1007/s11306-005-4810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. American Journal of Physiology Heart and Circulatory Physiology. 2012;303(11):H1273–H1282. doi: 10.1152/ajpheart.00492.2012. [DOI] [PubMed] [Google Scholar]
- Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. American Heart Journal. 2012;163(5):844–850. doi: 10.1016/j.ahj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease—Results from the psychophysiological investigations of myocardial ischemia study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- Simko F, Paulis L. Melatonin as a potential antihypertensive treatment. Journal of Pineal Research. 2007;42:319–322. doi: 10.1111/j.1600-079X.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. American Journal of Physiology. 1992;262:R689–R697. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Wei J, Krishnan KR, Karoly ED, Mitchell MW, O'Connor CM, et al. Metabolomic differences in heart failure patients with and without major depression. Journal of Geriatric Psychiatry and Neurology. 2010;23(2):138–146. doi: 10.1177/0891988709358592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Progress in Neurobiology. 2001;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. European Heart Journal. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- Tanu DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and reactive nitrogen species? Journal of Pineal Research. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Teague CR, Dhabhar FS, Barton RH, Beckwith-Hall B, Powell J, Cobain M, et al. Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague–Dawley rats. Journal of Proteome Research. 2007;6(6):2080–2093. doi: 10.1021/pr060412s. [DOI] [PubMed] [Google Scholar]
- Végh A, Papp JG, Semeraro C, Fatehi-Hasanabad Z, Parratt JR. The dopamine receptor agonist Z1046 reduces ischaemia severity in a canine model of coronary artery occlusion. European Journal of Pharmacology. 1998;344(2–3):203–213. doi: 10.1016/s0014-2999(97)01615-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011a;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nature Medicine. 2011b;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu H, McKenzie G, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nature Medicine. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda M, Kim HK, Kim N, Ko KS, Rhee BD, Han J. A matter of life, death and diseases: Mitochondria from a proteomic perspective. Expert Review of Proteomics. 2013;10(1):97–111. doi: 10.1586/epr.12.69. [DOI] [PubMed] [Google Scholar]
- Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacological Reviews. 2012;64(2):359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoxia Gaoa X, Zhenga X, Li Z, Zhoua Y, Suna H, Zhanga L, Guoa X, Dud G, Qina X. Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. Journal of Ethnopharmacology. 2011;137:690–699. doi: 10.1016/j.jep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. Journal of the American College of Cardiology. 2013;61(7):714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Reddy RD, Keshavan MS, Monstrose DM, Matson WR, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naïve patients with schizophrenia. Molecular Psychiatry. 2010;15(9):938–953. doi: 10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Ko CM, Ahn YS, Park KS, Choe KH, Yoo KJ, et al. Mechanism of decrease in heart rate by peripheral dopaminergic D2-receptors. Yonsei Medical Journal. 1994;35(4):411–419. doi: 10.3349/ymj.1994.35.4.411. [DOI] [PubMed] [Google Scholar]


