FIGURE 1.
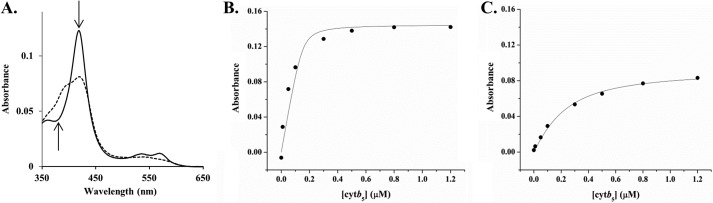
Determination of the dissociation constant Kd between cytb5 and wt-CYP2B4. A. Illustration of Type I spectral shift of CYP2B4 induced by cytb5: decrease at 420 nm and increase at 385 nm. Solid line, CYP2B4; dashed line, CYP2B4 bound with cytb5 (the absolute cytb5 spectrum is subtracted). B and C, titration of cytb5 into 0.3 μm wt-CYP2B4 in the presence (B) and absence (C) of 30 μm DLPC lipid bilayers. Both titrations were performed in 100 mm potassium phosphate buffer, containing 5% (w/v) glycerol, 0.3 μm benzphetamine, pH 7.4. The Kd values for wt-CYP2B4-cytb5 were determined to be 0.008 ± 0.015 μm in DLPC lipid bilayers (B) and 0.14 ± 0.02 μm in the absence of DLPC lipid bilayers (C).
