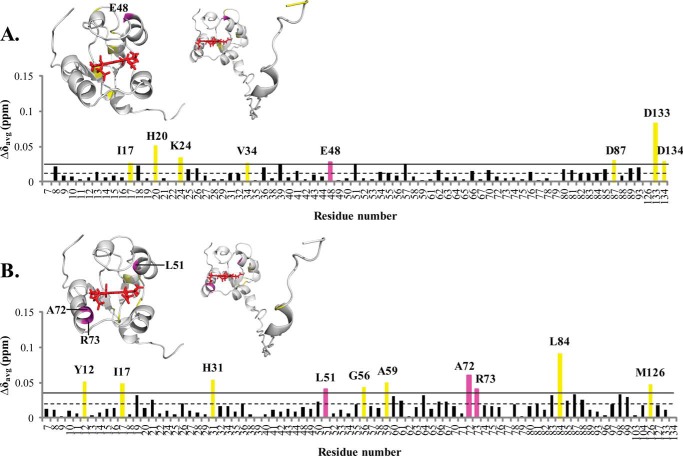
FIGURE 8.
Chemical shift perturbation of cytb5 resonances upon complex formation with tr-CYP2B4. As with wt-CYP2B4-cytb5 interactions, CSPs from tr-CYP2B4-cytb5 are also characterized by the small magnitude and wide dispersion, rendering accurate binding interface mapping impossible but allowing rough estimations of epitopes involved in tr-CYP2B4-cytb5 interactions. Weighted averages of chemical shift differences (Δδavg) for backbone amides of cytb5 upon interaction with tr-CYP2B4 at a 1:1 molar ratio in a lipid-free solution (A) and solution containing DLPC/DHPC isotropic bicelles (B) are plotted against cytb5 residue number. The dashed line represents the mean chemical shift change among all residues, and the solid line represents the mean chemical shift change plus one S.D. Residues with Δδavg above the solid line are considered to be the most affected among all of the cytb5 residues upon the addition of tr-CYP2B4. The residues most affected are highlighted in magenta for those located on the front face of cytb5 around the heme edge and yellow for those not in this area, both on the histogram and in the three-dimensional structures of cytb5 above each corresponding histogram. The structure of the soluble domain of cytb5 is shown on the left, and that of the full-length cytb5 is shown on the right.