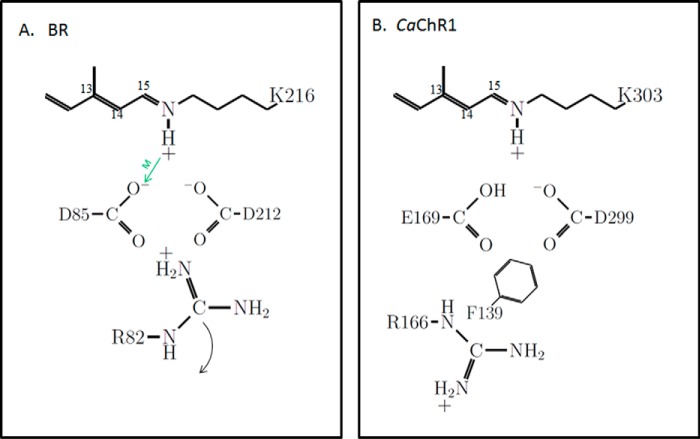
FIGURE 1.
Schematic showing key residues and their ionization states in the photoactive site of light-adapted BR and CaChR1. A, BR. The positioning of residues is guided by known high resolution x-ray structure (12). The green arrow indicates the role of Asp-85 as an SB proton acceptor during M412 formation (13). The curved arrow indicates repositioning of Arg-82 toward the extracellular side of the membrane that triggers proton release by the proton release complex (not shown). B, CaChR1. The positioning of the residues is guided by the high resolution x-ray structure of the C1C2 chimera (31). Ionization states are inferred from recent pH titrations on various CaChR1 mutants (8, 21). Phe-139 is replaced by a positively charged lysine in most high-efficiency ChRs (21).