Abstract
Organic anion-transporting polypeptides (Oatps) are an integral part of the detoxification mechanism in vertebrates and invertebrates. These cell surface proteins are involved in mediating the sodium-independent uptake and/or distribution of a broad array of organic amphipathic compounds and xenobiotic drugs. This study describes bioinformatics and biological characterization of 9 Oatp sequences in the Ixodes scapularis genome. These sequences have been annotated on the basis of 12 transmembrane domains, consensus motif D-X-RW-(I,V)-GAWW-X-G-(F,L)-L, and 11 conserved cysteine amino acid residues in the large extracellular loop 5 that characterize the Oatp superfamily. Ixodes scapularis Oatps may regulate non-redundant cross-tick species conserved functions in that they did not cluster as a monolithic group on the phylogeny tree and that they have orthologs in other ticks. Phylogeny clustering patterns also suggest that some tick Oatp sequences transport substrates that are similar to those of body louse, mosquito, eye worm, and filarial worm Oatps. Semi-quantitative RT-PCR analysis demonstrated that all 9 I. scapularis Oatp sequences were expressed during tick feeding. Ixodes scapularis Oatp genes potentially regulate functions during early and/or late-stage tick feeding as revealed by normalized mRNA profiles. Normalized transcript abundance indicates that I. scapularis Oatp genes are strongly expressed in unfed ticks during the first 24 h of feeding and/or at the end of the tick feeding process. Except for 2 I. scapularis Oatps, which were expressed in the salivary glands and ovaries, all other genes were expressed in all tested organs, suggesting the significance of I. scapularis Oatps in maintaining tick homeostasis. Different I. scapularis Oatp mRNA expression patterns were detected and discussed with reference to different physiological states of unfed and feeding ticks.
Keywords: Ixodes scapularis, Organic anion transporting polypeptides, Expression analyses
Introduction
Ixodes spp. ticks are among the most medically important tick species and transmit the majority of human tick-borne disease agents. A recent paper advocating for one-health solutions listed 17 tick-borne diseases (Dantas-Torres et al., 2012), 7 of which are vectored by Ixodes tick spp. In North America, 4 of the 9 reported human tick-borne disease agents, namely Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Babesia microti, and Powassan encephalitis virus, are vectored by Ixodes scapularis Say and Ixodes pacificus Cooley and Kohls (Dantas-Torres et al., 2012). Likewise, in Europe, the number of tick-borne pathogens transmitted by ticks of the genus Ixodes is larger. Ixodes ricinus L., distributed all over Europe, is the principal vector of B. burgdorferi sensu lato, A. phagocytophuilum, Anaplasma ovis, Coxiella burnetii, Francisella tularensis, Rickettsia helvetica, Rickettsia monacensis, Babesia divergens, tick-borne encephalitis virus (TBEV), Eyach virus, and Louping ill virus (Gould et al., 2001; Labuda and Randolph, 1999; Rehse-Küpper et al., 1976; Tomanović et al., 2013). In eastern Europe and throughout Asia stretching out to Japan, Ixodes persulcatus Schulze appears to be the most important Ixodes vector species transmitting highly pathogenic Far Eastern and Siberian subtypes of TBEV, B. burgdorferi sensu lato, Borrelia miyamotoi, A. phagocytophilum, Ehrlichia muris, B. microti, and several Rickettsia spp. (Alekseev et al., 2003; Chausov et al., 2010; Fukunaga et al., 1995; Inokuma et al., 2007; Shpynov et al., 2007).
The importance of Ixodes tick spp. in public health was the underlying rationale to sequence the I. scapularis genome (Hill and Wikel, 2005). The availability of I. scapularis genome data and several EST sequences has provided new resources for in-depth studies in tick biology. The expectation is that these studies will uncover weaknesses in tick biology that can be targeted for development of anti-tick vaccines and implicitly, prevention of human tick-borne diseases (Hill and Wikel, 2005; van Zee et al., 2007). We are interested in understanding the role(s) of organic anion transporting polypeptides in I. scapularis tick physiology. According to previously established nomenclature, abbreviations for human organic anion transporting polypeptides (OATP) are capitalized, while in other organisms it is presented in lower case (Oatp) (Hagenbuch and Meier, 2004). We have used this convention through the rest of this manuscript.
Since 1994 when the first organic anion transporting polypeptide was described (Jacquemin et al., 1994), this group of proteins has attracted considerable research attention in biomedicine. OATPs/Oatps are Na+-independent transmembrane transporters of amphipathic organic molecules, both of endogenous and exogenous origin, which is not only crucial in maintaining homeostasis, but an important function in drug absorption and disposition (Niemi, 2007). The list of substrates transported by human, rat, and mouse OATPs/Oatps include bile salts, hormones, eicosanoids, drugs, peptides, organic anions, and even some organic cations and toxins (Abe et al., 1999; Briz et al., 2002; Cui et al., 2001; Fujiwara et al., 2001; Huber et al., 2007; Kullak-Ublick et al., 1995; Lu et al., 2008; Mikkaichi et al., 2004a; van Montfoort et al., 1999). The proposed mechanism of transportation is of the rocker-switch type, with substrate molecules passing through the central positively charged pore (Meier-Abt et al., 2005).
Structurally, OATPs/Oatps are similar to organic anion transporters and organic cation transporters, consisting of 12 transmembrane domains (TM) and having an intracellular positioning of both termini (Roth et al., 2012). Distinguishing characteristics of OATPs/Oatps include conserved domain D-X-RW-(I,V)-GAWW-X-G-(F,L)-L positioned at the border between extracellular loop (EL) 3 and TM 6 (Hagenbuch and Meier, 2003), N-glycosylation sites in ELs 2 and 5 (Yao et al., 2012), and conserved cysteine amino acid residues in EL 5 that show similarity to Kazal-type serine protease inhibitors (Meier-Abt et al., 2005). All conserved cysteine amino acid residues in EL 5 normally form disulfide bonds and appear to be essential for function (Hänggi et al., 2006). Genes encoding OATPs/Oatps are classified into the solute carrier organic anion transporters gene group (SLCO). Hagenbuch and Meier (2004) established a new nomenclature and phylogenetic classification of OATP/SLCO as a superfamily, dividing previously described OATPs/Oatps into 12 families and further into subfamilies. Human and other mammalian sequences were classified into 6 families (OATP/Oatp1–6), while Oatp sequences in non-mammalian species were classified in the remaining 6 families (Hagenbuch and Meier, 2004).
Most of the data available for invertebrate Oatps comes from work with Drosophila species. A total of 8 putative Drosophila Oatp genes were identified and designated as 26F, 30B, 33Ea, 33Eb, 58Da, 58Db, 58Dc, and 74D, according to the chromosomal region where they are mapped (Torrie et al., 2004). Transporter 58Db has been linked to Drosophila resistance to ouabain, a cardiac glycoside known as a potent inhibitor of Na+/K+ ATPase. Na+/K+ ATPase is very important for Malpighian tubule (MT) function in insects. In Drosophila, protection of MT function against ouabain toxicity, even at very high concentrations, was attributed to Oatp 58Db (Torrie et al., 2004). Resistance to ouabain was also reported in several insect species (Gee, 1976; Neufeld and Leader, 1997) suggesting that Oatp detoxification function could be widespread in insects. Mulenga et al. (2008) performed molecular and biological characterization of Amblyomma americanum L. tick Oatp. Sequence analysis showed that this transporter possesses all features specific for the OATP/SLCO superfamily, while expression analysis demonstrated constant presence of its messenger RNA in different tick organs during the feeding process. Additionally, semi-quantitative RT-PCR analysis revealed significant changes in Oatp expression levels between tick organs during feeding. Gene silencing by RNAi caused smaller blood meals to be taken by A. americanum females, indicating that disrupting Oatp function will lead to decreased fertility (Mulenga et al., 2008).
The purpose of this study was to characterize and validate mRNA expression during tick feeding of Oatps in the I. scapularis genome. We show that, similar to mammals, the I. scapularis tick genome encodes a large Oatp family of at least 9 unique genes. Most importantly, all annotated I. scapularis Oatps are expressed during tick feeding. Like humans and rodents, which share OATP/Oatp orthologs, I. scapularis Oatp genes show remarkable conservation with Metastriata tick species.
Materials and methods
Data mining to identify I. scapularis Oatp sequences, bioinformatics, and phylogeny analyses
The National Center of Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov) was scanned for the presence of I. scapularis Oatp sequences using BlastX and BlastP search engines. Criteria for accepting sequences as part of the SLCO/OATP superfamily were presence of the characteristic D-X-RW-(I,V)-GAWW-X-G-(F,L)-L sequence and conserved cysteine amino acid residues in the extracellular loop (EL) 5. To compare I. scapularis Oatp sequences to other tick Oatp sequences, local Blast was used to scan I. scapularis Oatp sequences against local tick transcriptome databases. The databases of 45494 amino acid and 109,796 nucleotide sequences were assembled from tick transcriptomes in GenBank. Downloaded transcriptomes included I. scapularis (accession# PRJNA34667), Amblyomma maculatum Koch (PRJNA72241), A. americanum (PRJNA188113, PRJNA30813, PRJNA160), I. ricinus (PRJNA177622), Antricola delacruzi Estrada-Peña, Barros-Battesti and Venzal (PRJNA158141), Hyalomma marginatum Koch (PRJNA52401), Rhipicephalus microplus Canestrini (PRJNA82295), and Rhipicephalus pulchellus Gerstäcker (PRJNA170743). Additionally, an in-house A. americanum transcriptome from unfed and 24-h partially-fed male and female adult ticks, as well as salivary glands (SG) and midguts (MG) of ticks that fed for 48, 96 and 120 h was scanned for Oatp sequences (PRJNA226980). The downloaded tick transcriptome FASTA files were assembled into one file and then converted into a searchable database using the “make database” script at NCBI (http://www.ncbi.nlm.nih.gov/books/NBK1763/). Sequence alignment and analyses were performed with MacVector (MacVector Inc., Cary, NC, USA) and BioEdit (Hall, 1999) software. Prediction of N-glycosylation sites was performed using the NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/).
For phylogenetic analysis, the online software “Phylogeny.fr” was used (http://www.phylogeny.fr/version2_cgi/simple_phylogeny.cgi) (Dereeper et al., 2008) set to default parameters, with 100 replications for determining bootstrap values. Analysis was restricted to the EL5 region in the carboxy terminus, which is apparently important for OATP/Oatp function (Hänggi et al., 2006). EL5 domains in OATP/Oatp sequences of other ticks (A. americanum and R. pulchellus), of bloodsucking insects (Aedes aegypti, Anopheles gambiae, Culex quinquefasciatus, and Pediculus humanus corporis), and blood- and tissue-dwelling parasites (Brugia malayi, Loa loa, and Trichinella spiralis), and humans and rats were used in the analysis (Table 1).
Table 1.
Accession numbers of organic anion transporting polypeptide sequences used in phylogenetic analyses.
| Species | Accession numbers | Comments | Species | Accession numbers | Comments |
|---|---|---|---|---|---|
| Ixodes scapularis | XP002412161.1 | Direct submission | Amblyomma americanum | GAQI01000001a | Direct submission |
| XP002434179.1 | Direct submission | GAQI01000002 a | Direct submission | ||
| XP002400770.1 | Direct submission | GAQI01000003 a | Direct submission | ||
| XP002435666.1 | Direct submission | GAQI01000004 a | Direct submission | ||
| XP002414101.1 | Direct submission | GAQ01000005 a | Direct submission | ||
| XP002404592.1 | Direct submission | GAQI01000006 a | Direct submission | ||
| XP002415171.1 | Direct submission | GAQI01000007 a | Direct submission | ||
| XP002404594.1 | Direct submission | GAQI01000008 a | Direct submission | ||
| DAA34891.1 | Mulenga et al., 2008 | GAQI01000009 a | Direct submission | ||
|
|
|||||
| Loa loa | EFO23579.1 | Direct submissions | GAQI01000010 a | Direct submission | |
| EFO27139.1 | GAQI01000011 a | Direct submission | |||
| XP003140379.1 | GAQI01000012 a | Direct submission | |||
| ACH98103.1 | Mulenga et al., 2008 | ||||
|
| |||||
| Rhipicephalus pulchellus | JAA64227.1 | Direct submissions | Pediculus humanus corporis | EEB20468.1 | Direct submissions |
| JAA59396.1 | EEB18131.1 | ||||
| JAA59849.1 | EEB11548.1 | ||||
| JAA58190.1 | EEB20444.1 | ||||
|
| |||||
| Aedes aegypti | XP001659726.1 | Nene et al., 2007 | Culex quinquefasciatus | EDS45572.1 | Direct submission |
| XP001661188.1 | EDS45569.1 | ||||
| XP001658583.1 | EDS26845.1 | ||||
| XP001660407.1 | EDS34303.1 | ||||
| XP001660406.1 | EDS26844.1 | ||||
| XP001658582.1 | EDS34301.1 | ||||
|
| |||||
| Anopheles gambiae | XP001237849.2 | Mongin et al., 2004 | Trichinella spiralis | Mitreva et al., 2011 | |
| XP316669.4 | XP003380728.1 | ||||
| XP319187.4 | XP003379962.1 | ||||
| XP557860.3 | XP003375370.1 | ||||
| XP314819.4 | XP003377138.1 | ||||
| XP319188.4 | XP003375368.1 | ||||
|
| |||||
| Ascaris suum | ADY42330.1 | Wang et al., 2011 | Brugia malayi | EDP35822.1 | Ghedin et al., 2007 |
| ADY42805.1 | EDP32916.1 | ||||
| ADY40875.1 | EDP30755.1 | ||||
|
| |||||
| Homo sapiens | Rattus norvegicus | NP001257515.1 | Kakyo et al., 1999 | ||
| NP001139418.1 | Pizzagalli et al., 2002 | NP001014292.1 | Strausberg et al., 2002 | ||
| NP001138683.1 | Tamai et al., 2000 | NP445893.1 | Li et al., 2001 | ||
| NP037404.2 | Tamai et al., 2000 | NP110465.1 | Abe et al., 1998 | ||
| NP057438.3 | Tamai et al., 2000 | NP571981.1 | Noe et al., 1997 | ||
| NP006437.3 | Abe et al., 1999 | NP058807.1 | Jacquemin et al., 1994 | ||
| NP062818.1 | König et al., 2000 | NP570092.1 | Hagenbuch et al., 2002 | ||
| NP005621.2 | Lu et al., 1996 | NP542964.1 | Nishio et al., 2000 | ||
| NP066580.1 | Kullak-Ublick et al., 1995 | EDM11534.1 | Florea et al., 2005 | ||
| NP112220.2 | Hagenbuch and Meier, 2003 | EDM08519.1 | Florea et al., 2005 | ||
| NP851322.3 | Mikkaichi et al., 2004a | EDL91884.1 | Florea et al., 2005 | ||
| NP775759.3 | Suzuki et al., 2003 | EDL88808.1 | Florea et al., 2005 | ||
Sequences of in-house Amblyomma americanum transcriptome (PRJNA226980).
Tick dissections, RNA extraction, and cDNA synthesis
Unfed I. scapularis ticks for this study were purchased from Oklahoma State University. In our lab, ticks were routinely kept at favorable conditions (room temperature and >85% relative humidity). Ticks were fed on New Zealand White Rabbits according to the animal use protocol approved by Texas A & M University IACUC. A total of 18 unfed and 34 partially fed I. scapularis females was dissected. Partially fed females were manually detached at 4 different time points: 24 h (15 specimens), 72 h (10), 120 h (6), and 168 h (3) after feeding commenced. Ticks were washed in diethylpyrocarbonate (DEPC)-treated water, dried on a paper towel and dissected under a microscope as previously described (Mulenga et al., 2003, 2008). Freshly dissected tick organs: SG, MG, MT, ovaries (OV), and synganglion (SY), as well as carcass (CA), representing the tick remnants after removing tick organs, were placed in Trizol reagent (Invitrogen, Carlsbad, CA, USA). Dissected materials were pooled in 1 ml of Trizol according to time point and tissue type, homogenized, and stored at −80°C until total RNA extraction.
Total RNA from tick organs was extracted using the isopropanol precipitation method, according to detailed instructions provided by the Trizol manufacturer. Precipitated RNA was dissolved in nuclease-free water, and concentration was determined by measuring absorbance at 260 nm using the DU 640B spectrophotometer (Beckman Coulter Inc., Fullerton, CA, USA). Approximately 500 ng of total RNA was used to synthesize the first-strand cDNA template using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, SA, USA).
Temporal and spatial transcriptional analyses of I. scapularis Oatp during first five days of feeding
Semi-quantitative two-step RT-PCR was used to validate I. scapularis Oatp gene expression in different tick tissues during the first 5 days of the feeding process. In a preliminary analysis, titration PCR was done to determine the least number of PCR cycles at which PCR products were observable in all samples. Examining PCR products at 20, 25, 30, and 35 PCR cycles accomplished this. PCR primers and PCR conditions used are summarized in Tables 2 and 3, respectively. Routinely, PCR was done in 20-μL reaction volumes containing 10 μL of 2X MyTaq PCR master mix (Bioline USA Inc., Taunton, MA, USA), 2 μL of each primer (10 μM), and 90–150 ng of cDNA template for all tissues except for synganglion where we used up to 600 ng (Table 3). Preliminary analysis showed that some candidate Oatp genes were not expressed in all tissues. To validate low or no expression, we increased the amount of template tenfold in these tissues marked with asterisks in Table 3. An initial PCR activation step was carried out for 2 min at 95°C, followed by 33–40 cycles for 30 s at 95°C, 30 s of annealing at 60–70°C, and depending on amplicon length, 45–120 s of elongation at 72°C. A final extension step was performed at 72°C for 7 min. Following amplification, 10 μL of PCR product was applied to a 2% agarose gel containing 1 μg/mL of ethidium bromide and separated in TAE buffer at 50 V for 1 h. The amount of template cDNA used in PCR reactions and the number of amplification cycles were adjusted to obtain appropriate band density for further densitometry analyses (Table 3). Densitometric analyses were performed with ImageMaster TotalLab software (Nonlinear Dynamics Ltd., Newcastle, UK), using the option for automatic background removal. The relative PCR band density was normalized against tick actin PCR bands as previously described (Mulenga et al., 2008). The amplification of tick actin sequence was performed with 5′GGACAGCTACGTGGGCGACGAGG3′ and 5′CGATTTCACGCTCAGCCGTGGTGG3′ primers, using MyTaq Red Mix (Bioline USA Inc., Taunton, MA, USA) and PCR conditions as described in Table 3.
Table 2.
Primers used for expression analyses of identified Ixodes scapularis organic anion transporting polypeptides (Oatps).
| Forward primer 5′→3′ | Reverse primer 5′→3′ | Expected amplicon size (bp) | GenBank accession number of related nucleotide sequences | Designation of identified I. scapularis Oatp |
|---|---|---|---|---|
| gcgacggctgtgtacacgctgg | ctgctgttgcaggctggaggca | 815 | XM002400726 | IsOatp0726 |
| ggctcggaggcacggcctatta | gtactgcacggagccacaacga | 743 | XM002412114 | IsOatp2114 |
| cactggcgatgggccgtttgtg | caccgcgttcctcccacaccag | 810 | XM002412116 | IsOatp2116 |
| cccgtcacgaaaacgccttcatc | cggcatggcacggggagaagta | 732 | XM002414056 | IsOatp4056 |
| aactcttggaaacatcgccgtg | cgtcgtaaaagtctgtgacccg | 917 | XM002434134 | IsOatp4134 |
| catcatctgctcgctaatcccac | ccaggagaggaagaaaaagaggc | 700 | XM002404548 | IsOatp4548 |
| acccccgtgagctaccacgg | caacggggagcgctgtcaca | 559 | XM002404550 | IsOatp4550 |
| gggcgcctacaagtcgtacacc | agatgacgaacggcagagaggt | 377 | XM002415126 | IsOatp5126 |
| tcggaatccttgtggtagtttt | aaagtacgtggttcggtcatct | 626 | XM002435621 | IsOatp5621 |
Table 3.
Conditions for the second step of the semi-quantitative RT-PCR used for Ixodes scapularis Oatp gene expression analyzes.
| Targeted gene | TAa (°C) | ETb (seconds) | Amount of cDNA template used in the second step of RT-PCR (ng)
|
Number of cycles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG | MG | MT | OV | SY | CA | SG | MG | MT | OV | SY | CA | |||
| IsOatp0726 | 61 | 90 | 100 | 100 | 120 | 100 | 200 | 100 | 33 | 35 | 35 | 33 | 40 | 35 |
| IsOatp2114 | 70 | 60 | 100 | 100 | 120 | * | 200 | 100 | 35 | 38 | 35 | 35 | 35 | 35 |
| IsOatp2116 | 66 | 60 | * | 100 | * | * | 200 | * | 35 | 35 | 35 | 35 | 35 | 35 |
| IsOatp4056 | 67 | 60 | 100 | 100 | 120 | 100 | 200 | 100 | 35 | 35 | 35 | 35 | 33 | 35 |
| IsOatp4134 | 67 | 120 | 100 | * | * | * | * | * | 35 | 35 | 35 | 35 | 35 | 35 |
| IsOatp4548 | 60 | 90 | 100 | 100 | 90 | 100 | 600 | 100 | 35 | 35 | 35 | 35 | 40 | 35 |
| IsOatp4550 | 68 | 60 | * | * | * | 100 | * | * | 35 | 35 | 35 | 35 | 35 | 35 |
| IsOatp5126 | 68 | 45 | 100 | 100 | 120 | 150 | 400 | 100 | 35 | 35 | 35 | 40 | 40 | 35 |
| IsOatp5621 | 62 | 60 | 100 | 100 | 120 | 100 | 200 | 100 | 38 | 34 | 35 | 35 | 40 | 40 |
| Actin | 63 | 60 | 100 | 100 | 120 | 100 | 200 | 100 | 35 | 28 | 29 | 27 | 30 | 23 |
annealing temperature.
elongation time.
Amount of template in these reactions were increased up to 1 μg to validate low or no expression.
Cloning and DNA sequencing
To validate if sequences of amplified I. scapularis Oatp PCR fragments from semi-quantitative RT-PCR analysis were consistent with data in GenBank, DNA sequencing was conducted. Routinely, PCR fragments were cloned into pGEM-T cloning vectors (Promega, Madison, WI, USA) using TA cloning methods. Recombinant pGEM-T–I. scapularis Oatp plasmids were used to transform DH5α E. coli-competent cells. Plasmid DNA from liquid bacterial culture was extracted using Wizard Plus SV Mini-Prep Kit (Promega). Sequencing reactions were performed with the BigDye Terminator Cycle Sequencing Kit and run on a 3730xl sequencer (Applied Biosystems, Foster City, CA, USA).
Results
The I. scapularis genome encodes at least 9 unique Oatps
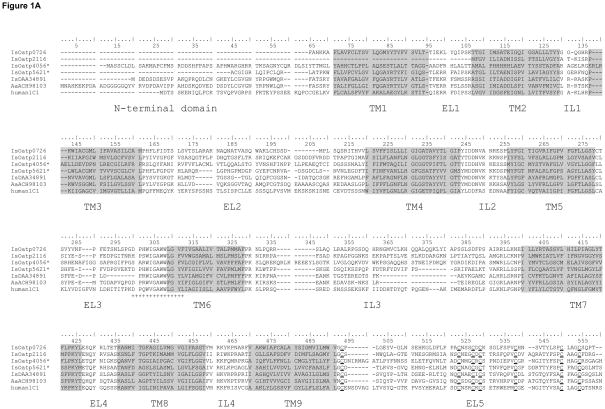
Data mining of I. scapularis sequence entries in GenBank identified 9 unique Oatp sequences (Table 4). Some of the I. scapularis Oatps aligned in Fig. 1A showed the typical signature of OATP/Oatp structure (Roth et al., 2012), 12 TM domains with large EL 2 and 5. Putative N-glycosylation sites in EL2 and EL5 characterize this superfamily of proteins (König et al., 2006). Likewise, visual inspection of sequences in Fig. 1A revealed putative N-glycosylation sites in EL5 of all I. scapularis Oatp sequences, while the presence of a putative N-glycosylation site in EL2 was predicted for IsOatp0726 (accession# XP002400770) only. Consistent with other OATPs/Oatps (Meier-Abt et al., 2005), I. scapularis Oatps have a Kazal-type serine protease inhibitor domain present in EL5 (Fig. 1A). Except for IsOatp4056 (XP002414101), I. scapularis Oatps are characterized by 11 consensus cysteine amino acid residues in EL5 (Fig. 1A). It is interesting to note that when scanned against GenBank entries, the IsOatp4056 EL5 cysteine amino acid residue pattern appeared similar to those described in Oatps from Tribolium castaneum (XP972698), Nasonia vitripennis (XP001605896), Bombus terrestris (XP003398370), and Apis florea (XP003695690) (Fig. 1B). Site-directed mutagenesis studies have shown that the 11 cysteine amino acid residues in EL5 are important in OATP/Oatp function (Hänggi et al., 2006). According to convention (Hagenbuch and Meier, 2004) IsOatp4134 (XP002434179) and IsOatp2114 (DAA34891), which show 54% amino acid identity (not shown), could belong to the same family, while most I. scapularis Oatps in this study could be classified into different families since intra-amino acid identity levels are ≤40%. It is important to note that while tick Oatp sequences retained sequence features that characterize the OATP/Oatp gene family, overall amino acid sequences identity levels between tick and mammalian sequences were low, ranging from 20% to 33% (not shown). We would like to note here that based on sequencing done in this study, sequence information was updated for IsOatp2114 (deletion of amino acid 297–310), IsOatp5621 (deletion of amino acid 352–354), and IsOatp4056 (27 amino acids inserted between positions 429 and 430) of original sequences. Updated I. scapularis Oatp nucleotide sequences were deposited in GenBank, and accession numbers KF768345, KF768347, and KF768346 assigned for IsOatp2114, IsOatp5621, and IsOatp4056.
Table 4.
The National Center for Biotechnology Information references of Ixodes scapularis organic anion transporting polypeptide sequences.
| Genomic sequence | Vector Base Gene ID | Reference nucleotide sequence | Protein sequence | Sequence length (aa) |
|---|---|---|---|---|
| NW002869999 (DS976794) | ISCW023852 | XM002400726 | XP002400770 (EEC20306) | 590 |
| NW002767795 (DS874590) | ISCW011144 | XM002412114 | XP002412159 (EEC14780) | 430 |
| NW002767795 (DS874590) | ISCW011146 | XM002412116 | XP002412161 (EEC14782) | 576 |
| NW002816190 (DS922985) | ISCW012022 | XM002414056 | XP002414101 (EEC17409) | 701 |
| NW002599711 (DS706506) | ISCW018349 | XM002434134 | XP002434179 (EEC05579) | 345 |
| NW002523651 (DS630446) | ISCW000594 | XM002404548 | XP002404592 (EEC01243) | 528 |
| NW002523651 (DS630446) | ISCW000596 | XM002404550 | XP002404594 (EEC01245) | 290 |
| NW002842230 (DS949025) | ISCW014692 | XM002415126 | XP002415171 (EEC18836) | 257 |
| NW002651402 (DS758197) | ISCW006481 | XM002435621 | XP002435666 (EEC08496) | 648 |
Fig. 1.
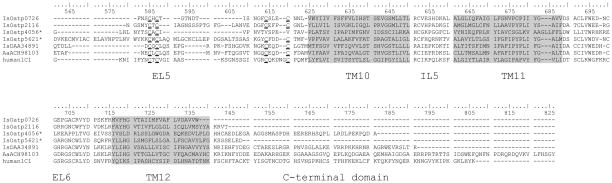

Multiple sequence alignment analysis: (A) alignment of Ixodes scapularis organic anion transporting polypeptide protein sequences. Asterisks denote sequences which are slightly changed from the GenBank original due to sequencing results obtained in this study (IsOatp5621*: amino acids at positions 352–354 are deleted; IsOatp4056*: 27 amino acids are inserted between positions 429 and 430 of the original sequence, accession numbers for updated nucleotide sequences are KF768347 and KF768346, respectively). Grey transmembrane domains (TM), extracellular loops (EL), and intracellular loops (IL) are identified according to Westholm et al. (2010). Double-underlined sequence represents SLCO/OATP family signature. Conserved cysteins in EL5 are marked with strong bottom line. (B) Alignment of EL 5 of IsOatp4056* and its orthologs from Tribolium castaneum, Nasonia vitripennis, Bombus terrestris, and Apis florea, with conserved cysteins designated with numbers 1–9. Conserved amino acids are highlighted in gray.
I. scapularis Oatps are not monolithic and have orthologs in other ticks
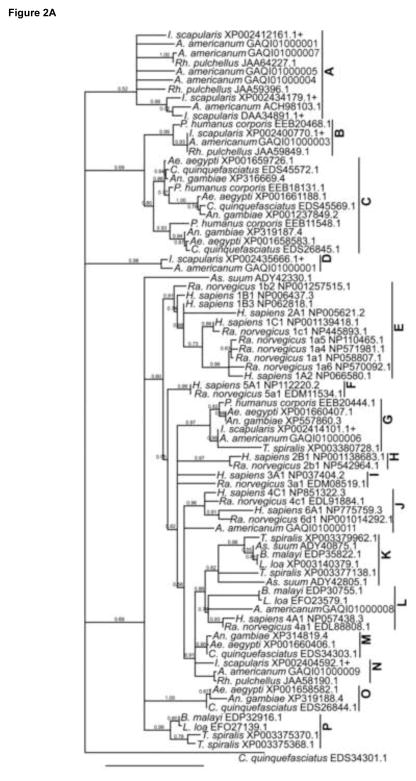
In addition to the 9 I. scapularis Oatps found in GenBank (above), scanning against other tick sequences in GenBank revealed tick Oatp sequences only from R. pulchellus. Additionally, inspection of our in-house A. americanum transcriptome showed 12 unique A. americanum Oatps. A total of 81 EL5 domains from blood-feeding and blood and tissue-dwelling parasites, as well as from mammals were used to construct the phylogeny tree in Fig. 2A. The 81 sequences segregated into 16 clusters, A–P, with 2 sequences from Ascaris suum (ADY42330) and C. quinquefasciatus (EDS34301) clustering alone. The overall trend from the phylogeny tree is that I. scapularis Oatp sequences did not cluster as a monolithic group, rather they segregated with Oatp sequences of other organisms, particularly ticks. Ixodes scapularis and other tick Oatp sequences segregated into 7 of the 16 clusters, A, B, D, G, J, L, and N. It is notable that in clusters B, G, J, and L, tick sequences clustered with those of other organisms. In cluster B, P. humanus corporis Oatp (EEB20468) segregated with 3 tick Oatp sequences: I. scapularis (XP002400770), A. americanum (GAQI01000003), and R. pulchellus (JAA59849). In cluster G, Oatps from blood-sucking arthropods A. americanum (GAQI01000006), I. scapularis (XP002414101), An. gambiae (XP557860), Ae. aegypti (XP001660407), and P. humanus corporis (EEB20444) clustered with an Oatp of a tissue-dwelling parasite, T. spiralis (XP003380728). In cluster J, A. americanum Oatp (GAQI01000011) clustered with mammalian OATPs/Oatps 6A1 (NP775759), 6d1 (NP001014292), and 4C1 (EDL91884, NP851322), while in cluster L, A. americanum Oatp (GAQI01000009) segregated with OATPs/Oatps from human and rat 4A1/a1 (NP057438, EDL88808), the filarial worm B. malayi (EDP30755), and the eye worm L. loa (EFO23579). Multiple sequence alignment analyses of I. scapularis, A. americanum, and R. pulchellus Oatp sequences identified putative tick Oatp orthologs. In cluster A, the IsOatp2116 (XP002412161) amino acid sequence was 67% identical to the A. americanum Oatp (GAQI01000002), while IsOatp4134 and IsOatp2114 amino acid sequences were 48 and 50% identical to A. americanum Oatp (ACH98103) (Mulenga et al., 2008), respectively (not shown). In cluster B, the IsOatp0726 amino acid sequence showed 77% (88% restricted to EL5 sequence) and 83% (87% restricted to EL5 sequence) amino acid identity to R. pulchellus Oatp (JAA59849) and A. americanum Oatp (GAQI01000003), respectively (Fig. 2B). It is notable that while the overall amino acid sequence identity of P. humanus corporis (EEB20468) to IsOatp0726 was 46%, it jumped to 54% when the comparison was restricted to the EL5 domain (not shown). In cluster D, IsOatp5621 (XP002435666) showed 64% amino acid sequence identity to A. americanum Oatp (GAQI01000001) (Fig. 2C), while in cluster N, IsOatp4548 (XP002404592) showed 77% and 78% amino acid sequence identity to A. americanum Oatp (GAQI01000009) and R. pulchellus Oatp (JAA58190), respectively (Fig. 2D).
Fig. 2.
Phylogenetic analyses based on extracellular loop 5 and transmembrane domains 8 and 10: (A) Ixodes scapularis organic anion transporting polypeptides and those from Rhipicephalus pulchellus and Amblyomma americanum ticks, human, rat, and other bloodsucking arthropods, blood- and tissue-dwelling parasites were used to construct phylogenetic tree. Ixodes scapularis sequences are marked with a plus sign. (B–D) Amino acid sequence alignment of tick Oatp orthologs from clusters B, D, and N. Conserved amino acids are highlighted in gray.
I. scapularis Oatp genes are responsive to tick feeding
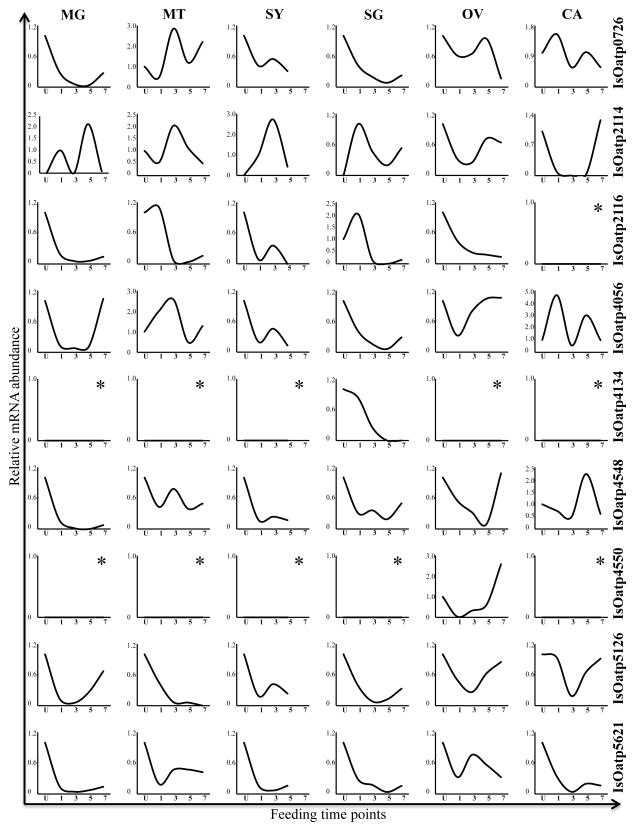
Titration semi-quantitative RT-PCR was used to confirm mRNA expression of 9 Oatp genes that are encoded in the I. scapularis genome (Table 2, Fig. 3). PCR products in Fig. 3 were amplified from ~90 to 150 ng of cDNA (Table 3). To validate low or not expression of the target, we attempted amplifying targets from ~1 μg of cDNA as summarized in Table 3. Routine PCR cloning, sequencing, and comparative sequence analysis validated the molecular identity of PCR bands in Fig. 3 (not shown). Visual inspection of PCR bands in Fig. 3 and normalized mRNA abundance in Fig. 4 summarize multiple mRNA expression patterns of the 9 I. scapularis Oatps during the first 5 days of tick feeding. Except for IsOatp4550 (XP002404594) and IsOatp4134, which were exclusively detected in the OV and SG, and IsOatp2116, which was not detectable in CA, all other candidate I. scapularis Oatp genes were expressed at detectable levels in all tissues that were investigated in this study (Fig. 3). IsOatp4550 and IsOatp4134 were not expressed even when template was added tenfold. Normalization of transcript levels in Fig. 4 revealed different mRNA expression patterns in different tick organs. The first pattern, an increase of mRNA expression in response to feeding, was observed for IsOatp4550 in OV. The second and most commonly observed pattern was a decrease of I. scapularis Oatp transcription levels in response to tick feeding activity. This pattern was associated with IsOatp2116 and IsOatp5621 in all organs, IsOatp0726 in all organs except MT, IsOatp4056 in SY, IsOatp4134 in SG, IsOatp4548 in MG, MT, SY, and SG, and IsOatp5126 (XP002415171) in MT and SY (Fig. 4). It should be noted that within the decreasing pattern, the drop in transcript levels between unfed and partially fed ticks was either precipitous or progressive. Transcript levels precipitously dropped within 24 h of tick feeding for IsOatp0726 in MG, IsOatp2116 in MG and SY, IsOatp4056 in SY, IsOatp4548 in MG and SY, IsOatp5126 in MT and SY, and IsOatp5621 in all organs except OV. In the case of IsOatp2116 in MT and SG, transcript levels did not change during the first 24 h of feeding. However, it precipitously dropped to an almost zero level by the third day of attachment. The expression level of IsOatp0726 in OV stayed relatively stable during the first 5 days of feeding, but then precipitously dropped by day 7. Progressive decrease in transcript levels was observed for IsOatp2116 in OV, IsOatp4134 in SG, and IsOatp0726 in SG and SY. In the third expression pattern, IsOatp0726 and IsOatp4056 in MT, and IsOatp2114 in MT, SY, and SG, showed an increase in mRNA expression levels during the early phase of tick feeding, reaching the peak of transcript expression levels at the 24–72 h time point, and thereafter a decrease by the 120 h or 168 h time points. In the fourth expression pattern, transcript levels of IsOatp2114 in CA, IsOatp4056 in MG and OV, IsOatp4548 in OV, and IsOatp5126 in MG, SG, OV, and CA were decreasing during the first 24–72 h of feeding, before rebounding to original levels during the 120–168 h feeding time point. IsOatp2114 in MG and IsOatp4056 in CA displayed a biphasic pattern; each of these genes had 2-peak expression levels during the 7-day feeding period that was investigated here. It is interesting to note that in the actin PCR, different amounts of template cDNA, as well as different number of amplification cycles, were used to obtain adequate actin bands for densitometric analyses, suggesting that different tick tissues express different levels of actin. This is not unique to this study. Previous studies conducted on different organisms, show that cytoplasmatic actin could have differential expression between tissues, but expression stays stable in the same tissue (Thellin et al., 1999; Trivedi and Arasu, 2005; Teng et al., 2012).
Fig. 3.
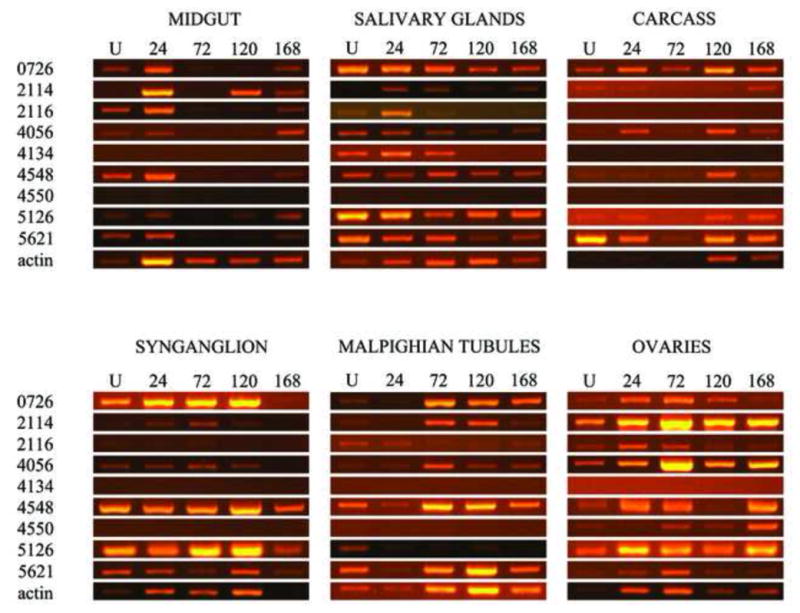
Semi-quantitative RT-PCR, showing expression of I. scapularis Oatps in different tick tissues before and during the feeding process.
Fig. 4.
Normalized mRNA abundance of 9 IsOatps during the feeding process (including unfed and 1-, 3-, 5-, and 7-days fed ticks) in salivary glands (SG), midgut (MG), Malpighian tubules (MT), ovaries (OV), synganglion (SY), and carcass (CA). Asterisks indicate graphs without detected expression.
Discussion
The importance of the OATP/Oatp gene family in the physiology of higher eukaryotes (König, 2011) and as a consequence, a potential druggable site (Kalliokoski and Niemi, 2009) prompted this study. Based on studies in humans (Mikkaichi et al., 2004b; Yao et al., 2012), rats (Mikkaichi et al., 2004b; Hagenbuch and Meier, 2004), mice (Cheng et al., 2005), Zebra fish (Popović et al., 2010), and the common fruit fly (Torrie et al., 2004), the OATP/Oatp gene family has multiple members. Observations in this study that the I. scapularis genome has at least 9 unique I. scapularis Oatp sequences and that the A. americanum transcriptome has 12 unique Oatp sequences, show that the size of the Oatp gene family in ticks fits the pattern in other organisms. It is noteworthy that the observation that I. scapularis Oatp genes had orthologs in A. americanum and R. pulchellus was comparable to observations among mammalian OATP/Oatp genes, where all human OATP genes have orthologs in rats and mice (Hagenbuch and Meier, 2004). It is interesting to note that in a parallel study, we observed similar clustering patterns of mosquito Oatp genes (unpublished). The role(s) of Oatp proteins in I. scapularis physiology is yet to be explored. However, given that I. scapularis Oatp proteins did not cluster as a monolithic group and that they have orthologs in other ticks suggests that I. scapularis Oatps are involved with yet unknown non-redundant biological processes that are evolutionarily conserved in other tick species.
One important problem of dealing with large protein families in parasite research is how to develop a prioritization plan, which gene comes first? In this study, we attempted to solve this problem by determining the relationships of I. scapularis tick Oatps to those of other blood feeding and blood- and tissue-dwelling parasites. Our reasoning was that parasites living in or on the same host environment could utilize related molecules to interact with the host, and that these shared molecules could represent important targets. The second consideration in our analyses was that we wanted to base the phylogeny on the EL5 domain, which has been shown to be important for OATP/Oatp function (Hänggi et al., 2006), and has been subject to different pressures during evolutionary change. From this perspective, it was interesting to find that certain tick Oatp sequences clustered with those from other parasites. It is particularly interesting that IsOatp0726 was 54% identical to the P. humanus corporis (EEB204468) EL5 domain. There is a possibility that both of these Oatp sequences transport substrates that are essential to the survival of both lice and ticks. It is important to note the lifestyle similarity between hard ticks (Sonenshine, 1993) and P. humanus corporis (Mehlhorn, 2008). Both organisms remain intimately attached onto the host for extended periods of time. From the perspective of discovery of anti-parasitic drugs, the prospect of parasite Oatps transporting the same substrate is appealing in that understanding how to block Oatp function in one parasite can be applied to multiple parasites. It was also notable from our phylogeny analysis that tick and other parasite Oatp sequences clustered with mammalian 4A1/a1, 4C1, and 6A1/a1 OATP/Oatps. The implication here could be that these tick and other parasite Oatps transport substrates that are similar to those transported by vertebrate OATPs/Oatps. This may not be far-fetched because as ticks and other parasites interact with their hosts, they may encounter host-derived toxic waste, which the parasite may need to eliminate. From the perspective of finding anti-parasitic targets, parasite Oatps that show similarity to vertebrate OATPs/Oatps may not be appealing targets due to potential cross-reactivity. Clearly, further experiments are needed to resolve observations from our phylogeny analysis.
Several lines of evidence have demonstrated that ticks differentially express genes during the different phases of feeding to regulate specific physiological processes (Rudenko et al., 2005; McNally et al., 2012). To begin characterizing the role(s) of I. scapularis Oatps in tick feeding physiology, an important goal in this study was to relate I. scapularis mRNA expression profiles to different phases of the tick feeding process. The majority of studies do this during the first 5 days of feeding (Franta et al., 2011; McNally et al., 2012) as was done in this study. Ticks spend the majority of their life cycle as free-living and a relatively small amount of time parasitizing their hosts (Sonnenshine, 1993). Tick feeding has been categorized into 3 broad phases, beginning with the preparatory feeding phase (PFP) during the first 24–36 h of feeding, when the tick attaches onto host skin, creates the feeding lesion, and transmission of some viruses occurs (Charrel et al., 2004). Subsequently the tick transitions into a slow feeding phase (SFP), which may last up to 7 days. During this phase, the tick begins transmission of most pathogens (Heyman et al., 2010), feeds in moderation, and grows more tissue to prepare for the rapid feeding phase (RFP), where it feeds to repletion (Sonenshine, 1993). Transcription profiling reported here covered the first 7 days of I. scapularis feeding, representing the PFP and SFP of the tick feeding process. With the exception of the IsOatp4550 gene, which showed an increase in expression level in OV in response to feeding, the majority of I. scapularis Oatp genes were either highly expressed in unfed ticks, and expression level decreases in response to feeding within the first 24 h or temporarily decreases during 24–72 h of feeding, but rebounded by day 7 of feeding. Speculatively, I. scapularis Oatp genes, which showed decrease in expression level in response to feeding could be associated with PFP or play no role(s) in facilitating tick feeding. Those that showed increase could be associated with subsequent tick feeding phases. Likewise, I. scapularis Oatps which were exclusively detected in one tick organ could be associated with specific functions in those organs. Although several tick transcriptomes are available in GenBank, data mining efforts in this study yielded Oatp sequences from R. pulchellus and I. scapularis only. The observation that the majority of I. scapularis Oatp genes were highly expressed in unfed ticks and during the first 24 h of feeding could explain the absence of Oatp transcripts in the majority of transcriptomes present in GenBank, as they were obtained using ticks that were partially fed for more than 3 days. Interestingly, the 12 unique Oatps discovered in this study from our in-house A. americanum transcriptome, were found at the unfed and 24 h fed time points (unpublished data).
Another interesting observation in this study is that with the exception of 2 I. scapularis Oatp genes, IsOatp4134 and IsOatp4550, which were exclusively detected in SG and OV, all other genes were expressed in all tested organs. Evidence in mammals shows that OATPs/Oatps that have a broad substrate range are widely distributed in multiple organs, while those with a restricted substrate range tend to be expressed in specific tissues (Mikkaichi et al., 2004b). Whether or not this is case in ticks remains to be investigated in future studies.
Acknowledgments
This work was in part supported by grant support from the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH) grant (AI081093 and AI1AI093858) to AM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. doi: 10.1074/jbc.273.35.22395. [DOI] [PubMed] [Google Scholar]
- Abe T, Kakyo M, Tokui T, Nagakomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Alekseev AN, Semenov AV, Dubinina HV. Evidence of Babesia microti infection in multi-infected Ixodes persulcatus ticks in Russia. Exp Appl Acarol. 2003;29:345–353. doi: 10.1023/a:1025841901909. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Cui Y, Klaassen CD. Prominent expression of xenobiotic efflux transporters in mouse extraembryonic fetal membranes compared with placenta. Drug Metab Dispos. 2008;36:1960–1970. doi: 10.1124/dmd.108.021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, Marin JJ. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–860. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Attoui H, Butenko AM, Clegg JC, Deubel V, Frolova TV, Gould EA, Gritsun TS, Heinz FX, Labuda M, Lashkevich VA, Loktev V, Lundkvist A, Lvov DV, Mandl CW, Niedrig M, Papa A, Petrov VS, Plysnin A, Randolph S, Süss J, Zlobin VI, De Lamballerie X. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10:1040–1055. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- Chausov EV, Ternovoi VA, Protopopova EV, Kononova JV, Konovalova SN, Pershikova NL, Romanenko VN, Ivanova NV, Bolshakova NP, Moskvitina NS, Loktev VB. Variability of the tick-borne encephalitis virus genome in the 5′ noncoding region derived from ticks Ixodes persulcatus and Ixodes pavlovskyi in western Siberia. Vector Borne Zoonot Dis. 2010;10:365–375. doi: 10.1089/vbz.2009.0064. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab Dispos. 2005;33:1062–1073. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny. fr: robust phylogenetic analysis for the non-specialists. Nucl Acids Res. 2008;36:W465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea L, Di Francesco V, Miller J, Turner R, Yao A, Harris M, Walenz B, Mobarry C, Merkulov GV, Charlab R, Dew I, Deng Z, Istrail S, Li P, Sutton G. Gene and alternative splicing annotation with AIR. Genome Res. 2005;15:54–66. doi: 10.1101/gr.2889405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franta Z, Sojka D, Frantova H, Dvorak J, Horn M, Srba J, Talacko P, Mares M, Schneider E, Craik CS, McKerrow JH, Caffrey CR, Kopacek P. IrCL1 – the haemoglobinolytic cathepsin L of the hard tick, Ixodes ricinus. Int J Parasitol. 2011;41:1253–1262. doi: 10.1016/j.ijpara.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. Identification of thyroid hormone transporters in humans: different molecules are involved in tissue-specific manner. Endocrinology. 2001;142:2005–2012. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- Gee JD. Fluid secretion by the malpighian tubules of tsetse fly Glossina morsitans: the effects of ouabain, ethacrynic acid and amiloride. J Exp Biol. 1976;65:323–332. doi: 10.1242/jeb.65.2.323. [DOI] [PubMed] [Google Scholar]
- Ghedin E, Wang S, Spiro D, Caler E, Zhao Q, Crabtree J, Allen JE, Delcher AL, Guiliano DB, Miranda-Saavedra D, Angiuoli SV, Creasy T, Amedeo P, Haas B, El-Sayed NM, Wortman JR, Feldblyum T, Tallon L, Schatz M, Shumway M, Koo H, Salzberg SL, Schobel S, Pertea M, Pop M, White O, Barton GJ, Carlow CK, Crawford MJ, Daub J, Dimmic MW, Estes CF, Foster JM, Ganatra M, Gregory WF, Johnson NM, Jin J, Komuniecki R, Korf I, Kumar S, Laney S, Li BW, Li W, Lindblom TH, Lustigman S, Ma D, Maina CV, Martin DM, McCarter JP, McReynolds L, Mitreva M, Nutman TB, Parkinson J, Peregrín-Alvarez JM, Poole C, Ren Q, Saunders L, Sluder AE, Smith K, Stanke M, Unnasch TR, Ware J, Wei AD, Weil G, Williams DJ, Zhang Y, Williams SA, Fraser-Liggett C, Slatko B, Blaxter ML, Scott AL. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, De Lamballerie X, Zanotto PMA, Holmes EC. Evolution, epidemiology and dispersal of flaviviruses revealed by molecular phylogenies. Adv Virus Res. 2001;57:71–103. doi: 10.1016/s0065-3527(01)57001-3. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Gao B, Meier PJ. Transport of xenobiotics across the blood-brain barrier. News Physiol Sci. 2002;17:231–234. doi: 10.1152/nips.01402.2002. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflügers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hall TA. BiEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hänggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol. 2006;70:806–817. doi: 10.1124/mol.105.019547. [DOI] [PubMed] [Google Scholar]
- Heyman P, Cochez C, Hofhuis A, Van Der Giessen J, Sprong H, Porter SR, Losson B, Saegerman C, Donoso-Mantke O, Niedrig M, Papa A. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther. 2010;8:33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis genome project: an opportunity for advancing tick research. Trends Parasitol. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfändler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, Folkers G, Meier PJ, Stieger B. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292:C795–806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Ohashi M, Jilintai Tanabe S, Miyahara K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, eastern Hokkaido, Japan. J Vet Med Sci. 2007;69:661–664. doi: 10.1292/jvms.69.661. [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na+-independent organic anion transporter. P Natl Acad Sci USA. 1994;91:133–137. doi: 10.1073/pnas.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakyo M, Unno M, Tokui T, Nakagomi R, Nishio T, Iwasashi H, Nakai D, Seki M, Suzuki M, Naitoh T, Matsuno S, Yawo H, Abe T. Molecular characterization and functional regulation of a novel rat liver-specific organic anion transporter rlst-1. Gastroenterology. 1999;117:770–775. doi: 10.1016/s0016-5085(99)70333-1. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J. Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. Handb Exp Pharmacol. 2011;201:1–28. doi: 10.1007/978-3-642-14541-4_1. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432–443. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Labuda M, Randolph SE. Survival of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentralbl Bakteriol. 1999;291(S33):513–524. doi: 10.1016/s0934-8840(99)80005-x. [DOI] [PubMed] [Google Scholar]
- Li JY, Boado RJ, Pardridge WM. Blood-brain barrier genomics. J Cereb Blood Flow Metab. 2001;21:61–68. doi: 10.1097/00004647-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song P, Klaassen CD. Characterization of organic anion transporting polypeptide 1b2-null mice: Essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol Sci. 2008;103:35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA (hPGT) J Clin Invest. 1996;98:1142–1149. doi: 10.1172/JCI118897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. Encyclopedia of Parasitology. 3. Springer Reference; 2008. [Google Scholar]
- McNally KL, Mitzel DN, Anderson JM, Ribeiro JM, Valenzuela JG, Myers TG, Godinez A, Wolfinbarger JB, Best SM, Bloom ME. Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick-Borne Dis. 2012;3:18–26. doi: 10.1016/j.ttbdis.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: Identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membrane Biol. 2005;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. P Natl Acad Sci USA. 2004a;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T. The organic anion transporter (OATP) family. Drug Metab Pharmacokin. 2004b;19:171–179. doi: 10.2133/dmpk.19.171. [DOI] [PubMed] [Google Scholar]
- Mitreva M, Jasmer DP, Zarlenga DS, Wang Z, Abubucker S, Martin J, Taylor CM, Yin Y, Fulton L, Minx P, Yang SP, Warren WC, Fulton RS, Bhonagiri V, Zhang X, Hallsworth-Pepin K, Clifton SW, McCarter JP, Appelton J, Mardis ER, Wilson RK. The draft genome of the parasitic nematode Trichinella spiralis. Nat Genet. 2011;43:228–235. doi: 10.1038/ng.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin E, Louis C, Holt RA, Birney E, Collins FH. The Anopheles gambiae genome: an update. Trends Parasitol. 2004;20:49–52. doi: 10.1016/j.pt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC, Strey O, Teel P. Molecular and biological characterization of the Amblyomma americanum organic anion transporter polypeptide. J Exp Biol. 2008;211:3401–3408. doi: 10.1242/jeb.022376. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Macaluso KR, Simser JA, Azad AF. The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem Mol Biol. 2003;33:911–919. doi: 10.1016/s0965-1748(03)00097-3. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naceira HF, Nusbaum C, O’leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld DS, Leader JP. Electrochemical characteristics of ion secretion in malpighian tubules of the New Zealand alpine weta (Hemideina maori) J Insect Physiol. 1997;44:39–48. doi: 10.1016/s0022-1910(97)00087-5. [DOI] [PubMed] [Google Scholar]
- Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- Nishio T, Adachi H, Nakagomi R, Tokui T, Sato E, Tanemoto M, Fujiwara K, Okabe M, Onogawa T, Suzuki T, Nakai D, Shiiba K, Suzuki M, Ohtani H, Kondo Y, Unno M, Ito S, Iinuma K, Nunoki K, Matsuno S, Abe T. Molecular identification of a rat novel organic anion transporter moat1, which transports prostaglandin D(2), leukotriene C(4), and taurocholate. Biochem Biophys Res Commun. 2000;275:831–838. doi: 10.1006/bbrc.2000.3377. [DOI] [PubMed] [Google Scholar]
- Noe B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. P Natl Acad Sci USA. 1997;94:10346–10350. doi: 10.1073/pnas.94.19.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Popović M, Zaja R, Smital T. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): phylogenetic analysis and tissue distribution. Comp Biochem Phys A. 2010;155:327–335. doi: 10.1016/j.cbpa.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Rehse-Küpper B, Casals J, Rehse E, Ackermann R. Eyach, an arthropod-borne virus related to Colorado tick fever virus in the Federal Republic of Germany. Acta Virol. 1976;20:339–342. [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Edwards MJ, Grubhoffer L. Differential expression of Ixodes ricinus tick genes induced by blood feeding or Borrelia burgdorferi infection. J Med Entomol. 2005;42:36–41. doi: 10.1093/jmedent/42.1.36. [DOI] [PubMed] [Google Scholar]
- Shpynov S, Fournier PE, Rudakov N, Tarasevich I, Raoult D. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann N Y Acad Sci. 2006;1078:378–383. doi: 10.1196/annals.1374.075. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Oxford University Press; USA: 1993. [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. P Natl Acad Sci USA. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, Abe M, Satoh F, Unno M, Nunoki K, Suzuki M, Hishinuma T, Goto J, Shimosegawa T, Matsuno S, Ito S, Abe T. Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol Endocrinol. 2003;17:1203–1215. doi: 10.1210/me.2002-0304. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Teng X, Zhang Z, He G, Yang L, Li F. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in four lepidopteran insects. J Insect Sci. 2012;12:60. doi: 10.1673/031.012.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotech. 1999;75:291–295. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Tomanović S, Chochlakis D, Radulović Ž, Milutinović M, Ćakić S, Mihaljica D, Tselentis Y, Psaroulaki A. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp Appl Acarol. 2013;59:367–376. doi: 10.1007/s10493-012-9597-y. [DOI] [PubMed] [Google Scholar]
- Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JAT. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA. 2004;101:13689–13693. doi: 10.1073/pnas.0403087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S, Arasu P. Evaluation of endogenous reference genes for real-time PCR quantification of gene expression in Ancylostoma caninum. Mol Biochem Parasitol. 2005;143:241–244. doi: 10.1016/j.molbiopara.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Van Monfort JE, Hagenbuch B, Fattinger KE, Müller M, Groothuis GM, Meijer DK, Meier PJ. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. J Pharmacol Exp Ther. 1999;291:147–152. [PubMed] [Google Scholar]
- Van Zee PJ, Geraci NS, Guerrero FD, Wikel SK, Stuart JJ, Nene VM, Hill CA. Tick genomics: the Ixodes genome project and beyond. Int J Parasitol. 2007;37:1297–1305. doi: 10.1016/j.ijpara.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Czech B, Crunk A, Wallace A, Mitreva M, Hannon GJ, Davis RE. Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Res. 2011;21:1462–1477. doi: 10.1101/gr.121426.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westholm DE, Marold JD, Viken KJ, Duerst AH, Anderson GW, Rumbley JN. Evidence of evolutionary conservation of function between the thyroxine transporter Oatp1c1 and major facilitator superfamily members. Endocrinology. 2010;151:5941–5951. doi: 10.1210/en.2010-0640. [DOI] [PubMed] [Google Scholar]
- Yao J, Hong W, Huang J, Zhan K, Huang H, Hong M. N-glycosylation dictates proper processing of organic anion transporting polypeptide 1B1. PLOS One. 2012;7:e52563. doi: 10.1371/journal.pone.0052563. [DOI] [PMC free article] [PubMed] [Google Scholar]