Figure 1.
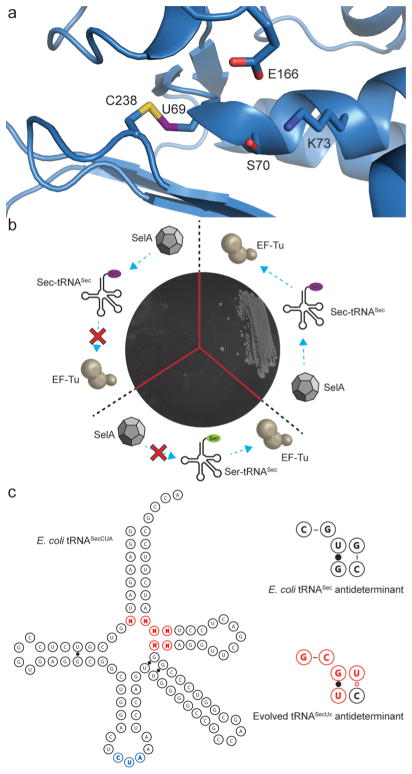
Selection of tRNAs capable of canonical incorporation of selenocysteine. (a) Representation of the NMC-A β-lactamase from Enterobacter cloacae (PDB: 1BUE) showing the engineered selenyl-sulfhydryl bond between residues 69 and 238 and its proximity to the catalytic site. (b) Validation of a β-lactamase reporter capable of discriminating serine from selenocysteine. Three NMC-A variants, C69X (TAG), C69, and C69S (clockwise from left), were constructed to determine the threshold for selection (<50 μg·mL−1). These approximate the following outcomes during selection: termination at the amber stop codon due to inefficient interaction with EF-Tu, impaired interaction with SelA promoting incorporation of serine, and efficient interactions with both SelA and EF-Tu resulting in incorporation of selenocysteine. (c) Representation of E. coli tRNASecCUA and the evolved antideterminant sequence. The antideterminant library is shown in red, and the CUA anticodon is shown in blue.