Figure 3.
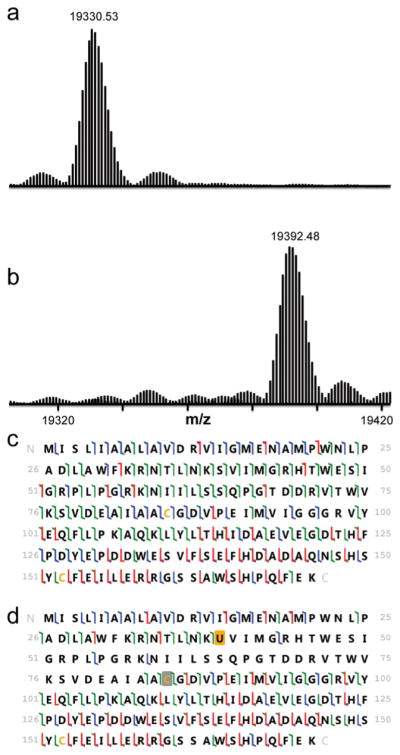
Intact mass and 193 nm UVPD results are shown for DHFR and seleno-DHFR produced using tRNASecUx. For the sequence information shown, disulfide-bound cysteines are shaded in gray, and selenocysteine is shaded in orange. When selenocysteine is located at position 39, it forms a selenyl-sulfhydryl bond with the cysteine at position 85. The different colors of slash marks represent the cleavage sites that lead to different N-terminal and C-terminal ions (green is used for a and x ions, blue is for b and y ions, and red is for c and z ions). (a) Expanded region of the deconvoluted mass spectrum of DHFR with serine at position 39 (expected average mass m/z 19331.72). (b) Expanded region of the deconvoluted mass spectrum of DHFR with selenocysteine incorporated at position 39 using tRNASecUx (expected average mass m/z 19392.66). (c) UVPD fragmentation map of DHFR P39S obtained for the 20+ charge state. (d) UVPD fragmentation map of DHFR with selenocysteine at position 39 (18+ charge state).
