Abstract
Improved detection programs and new therapies significantly improved the 5-year survival rate of women with breast cancer. However, some women still relapse and succumb to cancer because of metastatic disease. In particular, chronically depressed patients do not seem to benefit from newly developed treatments and present with shorter survival. The reason for this association is unclear, but recent cues from preclinical studies point to the possible contribution of neuroendocrine factors generated in response to chronic stress and depression. Retrospective clinical studies also suggest a beneficial effect of sympathetic blockade in terms of less advanced disease at diagnosis, lower cancer-specific mortality, longer disease-free survival and reduced metastasis development and tumor recurrence, especially in patients who have taken propranolol before diagnosis. Therefore, β-blockers or therapies normalizing sympathetic tone might be beneficial as early adjuvant therapies to limit skeletal metastases and growth and eventually to improve prognosis in patients with breast cancers.
Introduction
Although as many as 22% of women with stage IV breast cancer survive at least 5 years with optimal therapies, metastasis to distant organs remains a common and a still deadly complication associated with advanced breast cancers. As many as 70–80% of patients with breast cancer present with skeletal metastases. Metastatic breast cancer commonly arises months or years after treatment completion for early and localized breast cancer, and the risk of recurrence varies between patients, depending on the type of primary tumor, its stage at the time of initial diagnosis and likely on a number of intrinsic and extrinsic factors that affect host response. This condition cannot be cured and is currently managed by palliative interventions, focused on length and quality of life.
Cancer metastasis is overall an inefficient process through which cells acquire genetic and/or epigenetic characteristics that allow them to gain a proliferative advantage and higher migratory and survival properties. Although the cellular genetic and phenotypic make-up of a tumor is a major determinant of metastatic efficiency, oncogenic transformation is not sufficient for metastatic competence. A receptive microenvironment is indeed a prerequisite for establishing secondary tumor growth.1
The skeleton is a preferential organ for the homing of disseminated breast tumor cells. In the case of breast cancer, the interaction of cancer cells with the bone microenvironment is crucial for their ability to colonize this tissue and their subsequent survival, growth and promotion of the feed-forward cycle of bone destruction, first described by Mundy and collaborators.2,3,4 Thus, pharmacological interference with the microenvironmental support is an attractive strategy for repressing early bone metastasis. Toward that end, there is a need to identify the conditions and factors that make the bone microenvironment a hospitable tissue for breast cancer cell colonization and growth.
Understanding the early events and mediators promoting establishment of metastatic cells in distant organs, especially bone, is challenging in the setting of human clinical studies. This is because the steps involved occur at a time the primary tumor may not yet be detected, and also because one cannot easily detect low numbers of metastatic cells within the skeleton at the time they colonize this dense tissue. For lack of techniques to detect early metastatic cells within the skeleton, retrospective clinical studies can be useful in pointing to conditions or factors associated with reduced survival or increased recurrence, as a way to identify possible mechanisms priming the process of skeletal metastasis.
Long before epidemiological studies were performed, ancient medical writers such as Galen (AD 132) noticed that cancer was dependent on ‘black bile', which in Greek is synonymous of melancholia.5 This observation led to the idea that cancer incidence was associated with emotional factors, which was eventually scientifically refuted. However, a number of studies provided preclinical and clinical evidence that psychosocial factors may contribute to the progression of the disease and that the activation of neuroendocrine pathways may be involved. I will briefly review here the evidence supporting the contribution of sympathetic cues to the process of skeletal metastasis and discuss the potential of β-blockers as adjuvant drug during treatment of the primary cancer to prevent bone metastasis and possibly increase the prognosis of women with breast cancer.
Neuroendocrine Factors and Bone Metastasis
Chronic psychosocial stress and severe depression are two conditions that have been linked to increased breast cancer recurrence, reduced survival and poor prognosis.6,7,8,9,10,11,12 These conditions are particularly relevant to both breast and ovarian cancer, as women are more predisposed to depression than males.11,13,14 In addition, major depression is a frequent but under-recognized and under-treated condition among breast cancer patients.15,16 The use of β-blockers, on the other hand, has been associated with prolonged survival in women treated for breast cancer,17,18,19,20 as well as in patients affected with prostate,21,22 lung,23 ovarian24 and cutaneous melanoma.25,26 A commonality among these studies is that the conditions and drugs involved affect the sympathetic nervous system (SNS), whose activity is stimulated by chronic stress and depression and inhibited by β-blockers.
The SNS is part of the autonomic nervous system that controls involuntary body functions. Its main mediator is norepinephrine (NE), a neurotransmitter that acts via β-adrenergic receptors (βARs). These receptors are expressed broadly, including in bone cells and in cancer cells of multiple origins. The skeleton is richly innervated by sympathetic nerves and, as indicated above, a preferential organ for the metastasis of breast and prostate cancer cells. Studies in mice have shown that β1/2AR non-selective agonists, such as isoproterenol (ISO), or chronic unpredictable stress, known to stimulate sympathetic outflow and the Hypothalamic-Pituitary-Adrenal axis (HPA), alter bone remodeling and lead to bone loss. These effects are mediated by the stimulation of bone resorption and the inhibition of bone formation.27,28 On the other hand, mice receiving the β-blocker propranolol, a β1/2AR non-selective antagonist, gain bone, especially in conditions associated with high bone turnover.27 Accordingly, mutant mice lacking the β2AR display a late-onset increase in bone mass caused by high bone formation and low bone resorption.28,29 Adrenergic agonists promote osteoclastogenesis via action on osteoblasts and stimulation of Rankl (Receptor Activator of Nuclear Factor Kappa-B Ligand) expression.28 It also regulates hematopoietic stem cell bone marrow trafficking via CXCL12/SDF1 (stromal cell-derived factor-1),30 as well as insulin secretion via its effect on osteoblasts.30,31,32 Therefore, the bone marrow environment is innervated and responsive to sympathetic outflow or β1/2AR pharmacological stimulation, and the osteoblast lineage is critical to this response.
Remarkably, the cytokines involved in these actions of sympathetic nerves on bone cells are known to contribute to skeletal cancer cell metastasis.33,34,35 SDF1 for instance is expressed by osteoblasts and is considered a major cytokine for successful homing and survival of prostate and breast cancer cells in bone.36,37 High CXCR4 expression has also been correlated to poor clinical outcome in patients with breast cancers.38,39 Similarly, RANKL is expressed by osteoblasts and osteocytes (as well as T cells and chondrocytes) in bone. Its role in osteoclastogenesis is well established in the setting of bone remodeling as well as osteolytic bone destruction associated with breast metastatic tumors. It also stimulates melanoma, breast, prostate and lung cancer cell migration in vitro40,41 and melanoma cancer cell bone metastasis in vivo.35 Finally, RANK immunohistochemical positivity in combination with CXCR4 positivity identified breast cancer patients whose disease has a high probability to metastasize to bone.38 Collectively, these observations suggested that sympathetic activation, in response to chronic stress or depression, may transform the bone marrow environment into a favorable tissue for metastatic establishment.
Chronic Stress Favors Ovarian, Prostate and Breast Cancer Bone Metastasis
The aforementioned hypothesis is difficult to address through clinical studies; thus, several groups relied on preclinical models, which allow investigators to use a number of consistent and controlled experimental paradigms of stress or depression and to inoculate and track metastatic cancer cells at different stages of the metastatic process. Although these models are imperfect and plagued with several limitations, they can provide useful mechanistic information if results are interpreted with these limitations in mind and inform the design of future clinical intervention studies.
Studies focused on ovarian cancers suggested that ovarian cancer cells are directly influenced by sympathetic cues. In xenograph models based on the use of HeyA8 and SKOV3ip1 cells, sympathetic activation by restrain stress increased weight and vascular endothelial growth factor (VEGF)-mediated vascularization of intraperitoneal tumors.42 The observation that Adβr1 and Adrβ2-deficient ovarian cancer cell lines did not respond to mouse chronic immobilization stress with an increase in tumor weight indicated that this effect of sympathetic activation on primary tumor growth was mediated via the βARs expressed in cancer cells. Sood et al. also reported that chronic stress induced by daily restrain or ISO injections protected tumor cells from apoptosis upon loss of anchorage and detachment of the extracellular matrix in an orthotopic model of human ovarian cancer.43 Other studies indicated that βAR stimulation increases the expression of VEGF and angiogenesis in human breast ovarian and melanoma cancer cells42,44,45 and pro-metastatic matrix metalloproteinases and other inflammatory mediators in gastric tumors.46 αAR blockade was also shown to block the proliferative effect of catecholamines on breast cancer cells in vitro,47 although the stimulatory effect of catecholamine on breast cancer cells is not seen in all studies.48 These effects of sympathetic nerves on primary tumors are consistent with the known innervation of the prostate,49,50 ovaries51 and the skin.52 It is still unclear whether these direct effects of catecholamines on primary tumor cells have any repercussion on their ability to disseminate to secondary sites, although studies on prostate and ovarian cancer support a role for the β2AR and β3AR in tumor development and dissemination to distant organs.42,53 It is possible that the activation of sympathetic nerves in the primary tumor leads not only to increased tumor growth but also to remodeling of the host stroma to lead to a tissue, cell and cytokine profile that is favorable for cancer cell egress and dissemination to distant organs.
Studies related to breast cancer provided strong evidence for indirect effects of sympathetic cues on key host metastatic sites, including bones, lymph nodes and lungs, which are known to receive sympathetic innervation. A study by Sloan et al.,63 based on the use of 66cl4 breast cancer cells injected in the mammary fat pad of mice subjected to daily restrain or ISO injections, showed that sympathetic activation in mice stimulated the infiltration of activated macrophages (as well as myeloid-derived suppressor cells) into the parenchyma of the breast primary tumor and thereby induced a pro-metastatic gene expression signature that favors dissemination to distant organs. In vivo macrophage suppression, but not T-cell absence, inhibited metastasis of these cells to the lung under stress conditions, demonstrating the functional contribution of macrophages to this increase in lung metastasis. Whether a similar mechanism involving macrophages could be involved in metastasis to the skeleton remains to be determined. These studies showed that psychological factors, via activation of the SNS, can activate a metastatic switch within a growing primary tumor and that macrophages/monocytes could functionally extend the influence of sympathetic signaling beyond the distance of neurotransmitter diffusion from neuronal fibers, especially in tissues with nerve terminal densities lower than the adrenal or the central nervous system (like breast or bone), to help the dissemination of cancer cells to distant organs. Another study by Szpunar et al. showed that, although mouse 4T1 breast cancer cells do not express αARs and βARs, modulation of NE levels by NE reuptake inhibition with desipramine promoted tumor growth (but not metastasis to the lung), suggesting that sympathetic outflow can affect tumor growth without a direct input on cancer cells.54
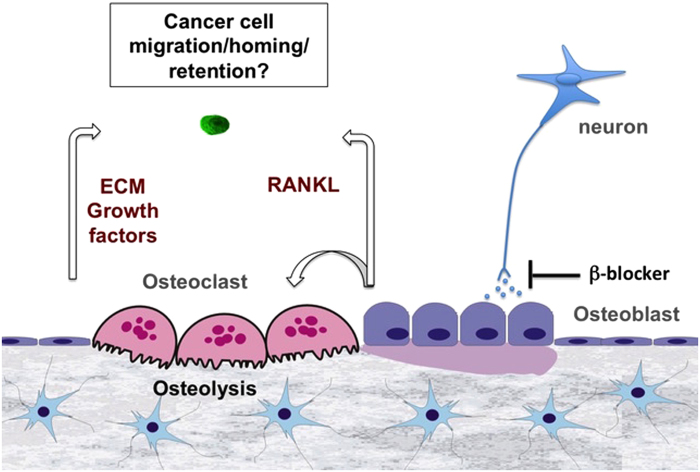
To investigate the mechanism by which sympathetic activation may alter the establishment of metastatic breast cancer cells in the skeleton, we have used intracardiac injections of the human osteotropic breast cancer cell line MDA-MB-231-VU in mice subjected to ISO treatment or daily immobilization stress (CIS). In this experimental setting, chosen for its focus on skeletal metastasis, we found that ISO and SNS activation by CIS stimulated the establishment of MDA-MB-231-VU cells within the skeleton.48 By subjecting mice to CIS or ISO treatment prior to MDA-MB-231-VU cell intracardiac inoculation, we were able to show that sympathetic nerves or βAR agonists promoted bone colonization via an indirect effect on the host stroma rather than via a direct effect on cancer cells. In this experimental paradigm, the number of lesions and metastatic foci was increased compared with control, whereas it was not if stimulation occurred after cancer cell inoculation. Tumor burden, however, was increased in both conditions compared with controls, most likely because CIS and ISO treatment promoted osteoclastogenesis, increased bone turnover and bone matrix growth factor release, thereby feeding the ‘vicious' feed-forward cycle of bone destruction. These effects were also observed with an osteotropic murine 4T1 clone in Balb-c mice, broadening these results to a distinct cancer cell line and to an immunocompetent host (unpublished data). Collectively, these data suggest that the affinity of breast cancer cells for the skeleton can be significantly increased by the action of sympathetic cues on this tissue.
The strong stimulatory effect of sympathetic activation and ISO treatment on the expression of Rankl in osteoblast cultures in vitro, as well as in bone in vivo, as well as the specificity of this increase in tissues where breast cancer cells metastasize, i.e. bone, liver and lung,48 led us to address whether this cytokine could mediate the stimulatory effect of sympathetic activation on breast cancer cell metastasis to the skeleton. Several studies demonstrated the direct contribution of RANKL to mammary tumorigenesis55,56,57 and melanoma bone metastasis.35 In the context of bone and breast cancer cells, we found that βAR agonists did not promote breast cancer cell proliferation in vitro and in a subcutaneous tumor growth model in vivo, thus supporting a main action on the host to promote skeletal establishment, rather than a direct effect on cancer cells. However, ISO stimulated the migration of MDA-231-VU cells in vitro, and this effect was inhibited by osteoprotegerin (OPG), the decoy receptor for RANKL, but not by AMD3100, a blocker of the receptor for SDF1/CXCL12.
The in vivo contribution of host-derived RANKL to breast cancer bone metastasis could not be investigated by using a classical systemic loss of function experiment using OPG for instance, as this approach would have affected osteoclastogenesis/bone turnover and the release of skeletal growth factors that could impact breast cancer cell establishment and proliferation in bone, in addition to the putative pro-migratory effect of RANKL on breast cancer cell homing to bone. Therefore, we chose to silence expression of the receptor for RANKL (RANK) in osteotropic MDA-MB-231-VU cells with the hypothesis that it should reduce their dissemination into the skeleton. This knockdown diminished MDA-MB-231-VU cell migration in vitro, did not affect cell proliferation and most importantly significantly decreased the establishment of these cells in bone in vivo following intracardiac injection and ISO pretreatment.48 These results suggest that sympathetic signals promote the skeletal establishment of breast cancer cells via a host-derived, RANKL-mediated mechanism (Figure 1). Of note is that nearly 40% of breast cancer metastatic tumors do not express RANK;58 hence, additional mechanisms likely contribute to breast cancer cell metastasis to bone. High levels of catecholamines and corticosteroids generated upon chronic stress for instance have immunosuppressive effects on natural killer (NK) cells, lymphocytes and macrophages, by affecting proliferation, differentiation or function of these cells.59,60,61,62 βAR loss of function experiments, targeting the β2AR in either osteoblasts, specific immune cell lineages or in cancer cells, will be one experimental strategy to determine the relative contribution of the major cell type(s) responding to the effect of sympathetic activation in the setting of bone metastasis. It will also be informative to determine whether tissue distribution of a non-osteotropic disseminating cancer clone could be altered by sympathetic activation in favor to skeletal sites versus soft tissues, such as liver or brain.
Figure 1.
Sympathetic neurons act on osteoblasts to stimulate breast cancer cell establishment in bone, via the pro-migratory and pro-resorptive effect of RANKL.
In conclusion, breast cancer metastatic cells may be affected by sympathetic cues in various manners, directly or indirectly, depending on their origin (breast, prostate, ovary, skin and so on) and stages of tumor development (primary growth, egress into the blood stream, survival, establishment at distant sites, dormancy and growth at these sites). Regardless of the mechanism, it appears from these preclinical studies that interventions aimed at reducing sympathetic nerve activity or downstream signals (including RANKL) may hold promise for limiting tumor cell dissemination to distant organs and thus for improving the prognosis of patients with cancers at risk for metastasis.
Stress, β-Blockers and Survival in Patients with Breast Cancer
An interesting observation in the course of these studies was that the β-blocker propranolol could prevent CIS-induced lung and bone metastasis of 66cl4 and MDA-MB-231-VU cells, respectively.48,63 These results support the specific role of catecholamines and βARs in these effects of chronic stress on lung and skeletal metastasis and also suggest that activation of the HPA axis is not preponderant in this stimulatory effect, although it certainly does not exclude its contribution. In that regards, the stimulatory effect of high-dose glucocorticoids on β2AR expression in osteoblasts for instance suggests that SNS and HPA activation may synergize to favor skeletal metastasis of breast cancer cells.64
The observation that propranolol reduced skeletal and lung establishment of breast cancer cells also suggests that β-blockers could have a beneficial effect clinically in women with breast cancer. This was indeed observed in a small number of retrospective studies.65 β-blockers are safe and well-characterized drugs, used clinically for the treatment of congestive heart failure and high blood pressure. Although these drugs do not seem to affect breast cancer incidence and tumorigenesis, a beneficial effect in terms of less advanced disease at diagnosis and lower cancer-specific mortality was observed in patients who have taken propranolol 1 year before diagnosis.19 Longer disease-free survival18 and reduced metastasis development and tumor recurrence were also observed in several studies,17,20 whereas some studies did not measure a significant effect of β-blockers on breast cancer recurrence or survival.66,67 In these latter studies, the majority of patients were taking β1AR-selective antagonists, which is different from all preclinical studies mostly based on effects via the β2AR and the use of β1/β2 non-selective antagonists. Finally, the observation of no association between post-diagnostic use of β-blockers and breast cancer-specific mortality further reinforces the notion that the early stages of metastatic dissemination represent the best window of opportunity for treatment to increase prognosis.68
Conclusion and Future Directions
The contribution of psychosocial factors to the clinical progression of breast cancer is supported by an increasing amount of preclinical data in mouse models and by retrospective clinical studies. Collectively, these studies support the pathophysiological relevance of the cross-talk between the neuronal and skeletal systems, provide new therapeutic opportunities to reduce or prevent metastatic spread in patients with low grade primary tumors and raise a number of interesting questions. For instance, experimental data support an effect of sympathetic signals on breast cancer bone metastasis via main effects on the host stroma; however, a direct effect of catecholamines on β2AR-positive breast cancer cells remains possible. This could affect early metastatic events such as tumor cell invasion, angiogenesis of the primary tumor, cancer cell survival or resistance to chemotherapy, as shown in studies related to other types of solid cancers.42,43,69,70,71,72,73,74,75 A stromal-mediated mechanism also implies that other types of metastatic cancers may be influenced by depression, chronic stress and subsequent sympathetic activation.42,70,71,72,75 The relative contribution of activation of the HPA axis and adrenal-derived epinephrine (versus nerve-derived NE) to bone remodeling and metastasis remains to be better characterized. As not every breast cancer cell line or tumor biopsy expresses the RANKL receptor (RANK), it will be important to determine whether and how RANK expression/signaling is regulated in cancer cells at the various stages of the metastatic process and to investigate the possible existence of additional mechanisms by which sympathetic activation may stimulate tumor metastasis. The molecular mechanism whereby RANKL promotes cancer cell migration also remains unclear. In addition, it is unknown whether the inhibitory effect of β2AR blockade on bone and lung metastasis observed in preclinical models contributes to the increase in relapse-free survival observed in patients with breast cancer who were taking β-blockers before breast cancer diagnosis. Lastly, the stimulatory effect of sympathetic activation on the production of inflammatory cytokines (tumor necrosis factor-α, interleukin-1, interleukin-6 and interferon-γ) that signal in neurons of the central nervous system controlling energy metabolism is also to consider in the context of cachexia seen in patients with metastatic cancers. Collectively, these studies warrant further investigations to address the potential of β-blockade or behavioral therapies to limit breast cancer spread to distant organs and to increase the prognosis of women diagnosed with breast cancer.
Acknowledgments
This work is supported by grant R01CA168717 from the NIH/NCI. The author acknowledges the valuable input from Dr S Park, Dr J Sterling and Dr A Zijlstra during these studies.
Footnotes
The author declares no conflict of interest.
References
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3: 537–549. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003; 97: 834–839. [DOI] [PubMed] [Google Scholar]
- Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone 48: 6–15. [DOI] [PubMed] [Google Scholar]
- Bryan J. Influence of mental depression on the development of malignant disease. Trans Am Surg Assoc 1901; 19: 685–688. [Google Scholar]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008; 5: 466–475. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003; 54: 269–282. [DOI] [PubMed] [Google Scholar]
- Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. Br Med J 2005; 330: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K. Mapping pathways from stress to cancer progression. J Natl Cancer Inst 2008; 100: 914–915 7. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M et al. Depression and stress reactivity in metastatic breast cancer. Psychosom Med 2006; 68: 675–683. [DOI] [PubMed] [Google Scholar]
- Vahdaninia M, Omidvari S, Montazeri A. What do predict anxiety and depression in breast cancer patients? A follow-up study. Social Psychiatry Psychiatr Epidemiol 45: 355–361. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Loving TJ, Robles TF, Kiecolt-Glaser JK. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain Behav Immun 2003; 17: S109–S111. [DOI] [PubMed] [Google Scholar]
- Lueboonthavatchai P. Prevalence and psychosocial factors of anxiety and depression in breast cancer patients. J Med Assoc Thai 2007; 90: 2164–2174. [PubMed] [Google Scholar]
- Mehnert A, Koch U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology 2007; 16: 181–188. [DOI] [PubMed] [Google Scholar]
- Hardman A, Maguire P, Crowther D. The recognition of psychiatric morbidity on a medical oncology ward. J Psychosom Res 1989; 33: 235–239. [DOI] [PubMed] [Google Scholar]
- Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA et al. Major depression after breast cancer: a review of epidemiology and treatment. Genl Hosp Psychiatry 2008; 30: 112–126. [DOI] [PubMed] [Google Scholar]
- Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010; 1: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011; 29: 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 2011; 29: 2635–2644. [DOI] [PubMed] [Google Scholar]
- Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B et al. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 2013; 140: 567–575. [DOI] [PubMed] [Google Scholar]
- Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 2013;. [DOI] [PubMed] [Google Scholar]
- Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013; 73: 250–260. [DOI] [PubMed] [Google Scholar]
- Wang HM, Liao ZX, Komaki R, Welsh JW, O'Reilly MS, Chang JY et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013; 24: 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 2012; 127: 375–378. [DOI] [PubMed] [Google Scholar]
- De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011; 171: 779–781. [DOI] [PubMed] [Google Scholar]
- Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH et al. β-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20: 2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002; 111: 305–317. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005; 434: 514–520. [DOI] [PubMed] [Google Scholar]
- Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med 2011; 208: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006; 124: 407–421. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Kajimura D et al. An Osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann NY Acad Sci 2009; 1173: E20–E30. [DOI] [PubMed] [Google Scholar]
- Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 2009; 30: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002; 62: 1832–1837. [PubMed] [Google Scholar]
- Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother 2006; 60: 273–276. [DOI] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006; 440: 692–696. [DOI] [PubMed] [Google Scholar]
- Wang N, Docherty FE, Brown HK, Reeves KJ, Fowles AC, Ottewell PD et al. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res 2014; 29: 2688–2696. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56. [DOI] [PubMed] [Google Scholar]
- Ibrahim T, Sacanna E, Gaudio M, Mercatali L, Scarpi E, Zoli W et al. Role of RANK, RANKL, OPG, and CXCR4 tissue markers in predicting bone metastases in breast cancer patients. Clin Breast Cancer 2011; 11: 369–375. [DOI] [PubMed] [Google Scholar]
- Hung CS, Su HY, Liang HH, Lai CW, Chang YC, Ho YS et al. High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases. Tumour Biol 2014; 35: 1581–1588. [DOI] [PubMed] [Google Scholar]
- Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate 2008; 68: 92–104. [DOI] [PubMed] [Google Scholar]
- Chen LM, Kuo CH, Lai TY, Lin YM, Su CC, Hsu HH et al. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J Cell Biochem 2011; 112: 933–941. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12: 939–944. [DOI] [PubMed] [Google Scholar]
- Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 2010; 120: 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kang JH, Jeong KJ, Lee J, Han JW, Choi WS et al. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int J Cancer 2011; 128: 2306–2316. [DOI] [PubMed] [Google Scholar]
- Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 2009; 23: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Liu D, Duan H, Han C, Wei B, Qian L et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer 2010; 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez SM, Pignataro O, Luthy IA. Alpha2-adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res Treat 1999; 55: 41–49. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol 2012; 10: e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CW, Xie JH, Ventura S. Age-related changes in the innervation of the prostate gland: implications for prostate cancer initiation and progression. Organogenesis 2013; 9: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather JN, Lau WA, Mitchelson F, Ventura S. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol 2000; 20: 193–206. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Russell P, Black K, Barrera H, Dubinovsky S, Markham R et al. Nerve fibers in ovarian endometriomas. Fertil Steril 2010; 94: 1944–1947. [DOI] [PubMed] [Google Scholar]
- Donadio V, Incensi A, Giannoccaro MP, Cortelli P, Di Stasi V, Pizza F et al. Peripheral autonomic neuropathy: diagnostic contribution of skin biopsy. J Neuropathol Exp Neurol 2012; 71: 1000–1008. [DOI] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341: 1236361. [DOI] [PubMed] [Google Scholar]
- Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013; 6: 1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468: 103–107. [DOI] [PubMed] [Google Scholar]
- Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER et al. Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802. [DOI] [PubMed] [Google Scholar]
- Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE 2011; 6: e19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M, Falk A, Rohne A, Wagner TO, Jacobs R, Tewes U et al. Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. J Clin Immunol 1993; 13: 344–351. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Mokyr MB, Graf LH Jr., Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J Immunol 1999; 163: 2492–2499. [PubMed] [Google Scholar]
- Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol 2000; 67: 553–558. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann NY Acad Sci 2002; 966: 290–303. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 2010; 70: 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. beta2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 2011; 152: 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Song T, Kim TH, Choi JK, Park JY, Yoon A et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol 2014; 140: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol 2011; 72: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen GV, Ganz PA, Cole SW, Pedersen LA, Sorensen HT, Cronin-Fenton DP et al. Use of beta-Blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish Nationwide Prospective Cohort Study. J Clin Oncol 2013; 31: 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. Int J Epidemiol 2013; 42: 1852–1861. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res 2003; 9: 4514–4521. [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH et al. Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Res 2006; 12: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 2006; 66: 10357–10364. [DOI] [PubMed] [Google Scholar]
- Palm D, Lang K, Niggemann B, TLt Drell, Masur K, Zaenker KS et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 2006; 118: 2744–2749. [DOI] [PubMed] [Google Scholar]
- Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem 2007; 282: 14094–14100. [DOI] [PubMed] [Google Scholar]
- Yang EV, Bane CM, MacCallum RC, Kiecolt-Glaser JK, Malarkey WB, Glaser R. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol 2002; 133: 144–150. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma QY, Hu HT, Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther 2010; 10: 19–29. [DOI] [PubMed] [Google Scholar]