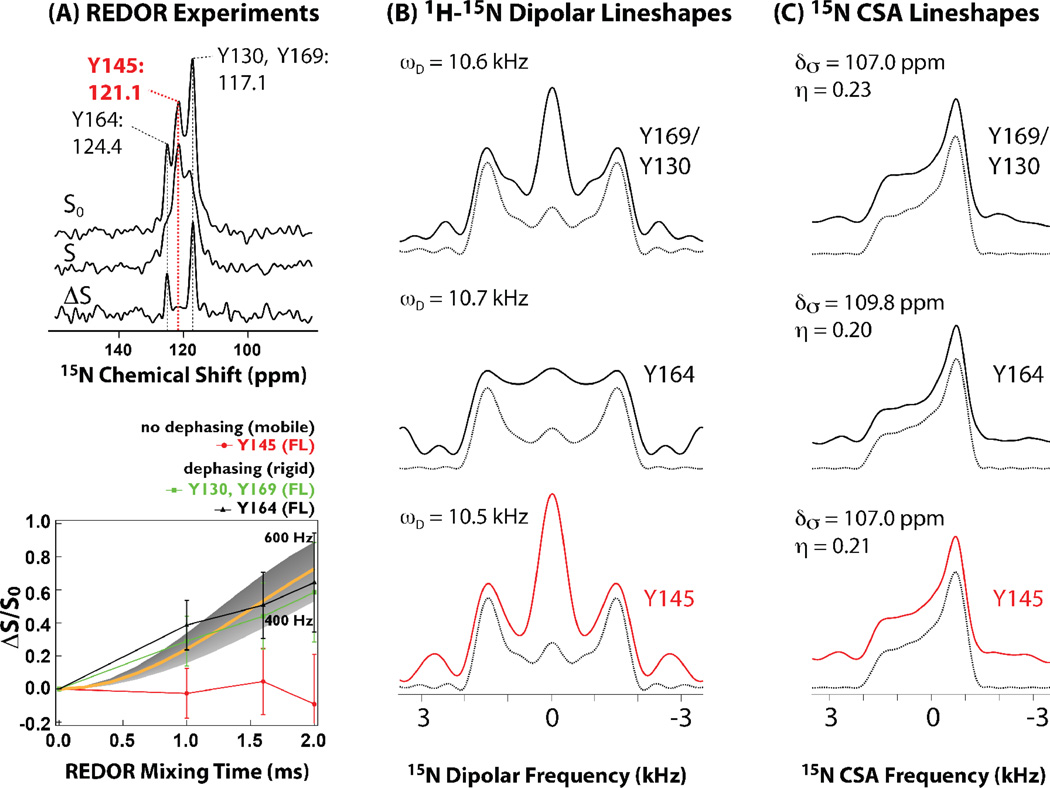
Figure 5.
A) 15N{13C} REDOR spectra (top) and dephasing curves (bottom) for conical assemblies of full-length HIV-1 CA protein. The lack of dipolar dephasing for Y145 in all constructs indicates millisecond timescale dynamics, which average the 15N-13C dipolar coupling. B) 1H-15N dipolar experimental and best-fit line shapes for U-13C,15N-Tyr labeled full-length HIV-1 CA protein assemblies of conical morphology. Dipolar coupling constants that were extracted by numerical simulations show that each Tyr residue is rigid on the time scale measured experimentally. C) 15N CSA line shapes for U-13C,15N-Tyr labeled full-length HIV-1 CA protein assemblies of conical morphology. Experimental and best-fit line shapes for each residue are shown. Each Tyr residue exhibits rigidity on the time scale measured experimentally. Adapted with permission from Byeon et al., J. Am. Chem. Soc., 2012, 134, 6455–6466. Copyright 2012 American Chemical Society.