Table 1.
Evaluation of Reaction Conditionsa
| |||
|---|---|---|---|
|
| |||
| Entry | Solvent | Additive | % Yield of 2ab |
| 1 | t-AmylOH | no | 45 |
| 2 | t-AmylOH | TEMPO | 40 |
| 3c | t-AmylOH | no | N.R. |
| 4 | Toluene | no | 30 |
| 5 | CH3CN | no | 35 |
| 6 | 1,4-Dioxane | no | 50 |
| 7 | DCE | no | 43 |
| 8 | DMF | no | 40 |
| 9 | CF3CH2OH | no | 60 |
| 10 | HFIP | no | 76(86)d |
| 11 | DCE | TFA | 75 |
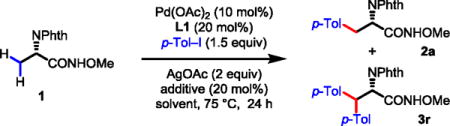
Conditions: Substrate 1 (0.1 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.2 mmol), p-Tol–I (0.15 mmol), Ligand L1 (20 mol%), solvent (1.0 mL), 75 °C, 24 h.
Determined by 1HNMR analysis of the crude product using CH2Br2 as an internal standard, and the yield is based on the amide and ester.
Reaction run at 60 °C.
Combined 76% mono- and 10% di-arylated products determined by crude 1HNMR.
