Table 11.
Arylation of Other Amino Acids and Carboxylic Acidsa
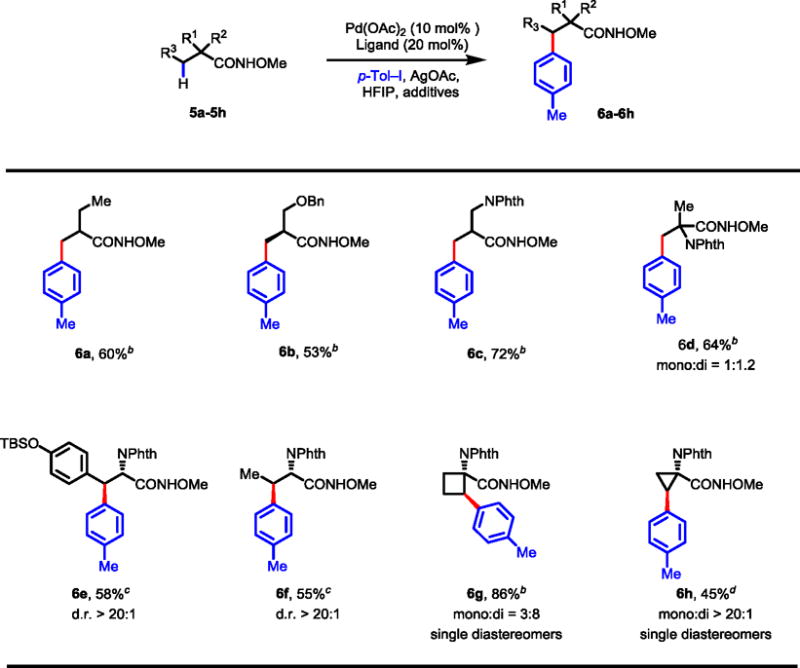
|
Isolated yields are shown.
Conditions: Substrate (0.2 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.4 mmol), p-Tol–I (0.3 mmol), 2-picoline (20 mol%), HFIP (2.0 mL), 80 °C, 24 h.
Conditions: Substrate (0.1 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.2 mmol), p-Tol–I (0.3 mmol), 2,6-lutidine (20 mol%), NaH2PO4•H2O (0.3 mmol), HFIP (1.0 mL), 90 °C, 36 h.
Substrate (0.1 mmol), Pd(OAc)2 (15 mol%), AgOAc (0.2 mmol), p-Tol–I (0.3 mmol), L1 (30 mol %), TFA (20 mol %), DCE (1.0 mL), 85 °C, 36 h.
