Highlights
-
•
Neurofeedback training of motor cortex shortens reaction times.
-
•
Self-regulation of parahippocampal cortex activity interferes with memory encoding.
-
•
Differential neurofeedback reveals double dissociation between neurofeedback target areas.
Keywords: Memory, Motor performance, Neurofeedback, Brain imaging, Functional magnetic resonance imaging (fMRI), Real-time fMRI, Self-regulation, Brain training
Abstract
Task performance depends on ongoing brain activity which can be influenced by attention, arousal, or motivation. However, such modulating factors of cognitive efficiency are unspecific, can be difficult to control, and are not suitable to facilitate neural processing in a regionally specific manner. Here, we non-pharmacologically manipulated regionally specific brain activity using technically sophisticated real-time fMRI neurofeedback. This was accomplished by training participants to simultaneously control ongoing brain activity in circumscribed motor and memory-related brain areas, namely the supplementary motor area and the parahippocampal cortex. We found that learned voluntary control over these functionally distinct brain areas caused functionally specific behavioral effects, i.e. shortening of motor reaction times and specific interference with memory encoding. The neurofeedback approach goes beyond improving cognitive efficiency by unspecific psychological factors such as attention, arousal, or motivation. It allows for directly manipulating sustained activity of task-relevant brain regions in order to yield specific behavioral or cognitive effects.
1. Introduction
Perception, memory, and performing a motor task depend on specific patterns of brain activity. These patterns of brain activity can be divided into transient activity elicited by the stimuli or events, and sustained activity that precedes the stimuli/events. Recent evidence indicates that both pre- and post-stimulus activity contribute to task performance (Arieli, Sterkin, Grinvald, & Aertsen, 1996; Boly et al., 2007; Fox & Raichle, 2007; Fox, Snyder, Vincent, & Raichle, 2007; Hesselmann, Kell, Eger, & Kleinschmidt, 2008a; Hesselmann, Kell, & Kleinschmidt, 2008b; Ress, Backus, & Heeger, 2000). While the latter is largely determined by the stimulus characteristics itself, the former can be modulated by attention, arousal, and motivation (Broadbent, 1971; Freeman, 1933; James, 1890; Wundt, 1882). Although such modulating factors play an important role in task performance, they are rather general factors of cognitive efficiency that cannot facilitate regionally specific brain processes.
Here, we manipulated sustained brain activity in circumscribed brain regions by using real-time functional magnetic resonance imaging (fMRI) based neurofeedback. Rather than modulating sustained pre-stimulus activity in an unspecific way (e.g. via arousal), this new approach allowed us to train participants to voluntarily ‘clamp’ pre-stimulus levels of regionally specific brain activity at high or low levels. Until now, neurofeedback was mainly used to train self-regulation of autonomic functions or of specific electroencephalography (EEG) components, in order to communicate with severely paralyzed patients (Birbaumer et al., 1999; Birbaumer, Murguialday, & Cohen, 2008; Kübler, Kotchoubey, Kaiser, Wolpaw, & Birbaumer, 2001), to suppress epileptic activity (Kotchoubey et al., 2001; Sterman & Egner, 2006; Tan et al., 2009), or to treat symptoms of attention deficit hyperactivity disorder (Fuchs, Birbaumer, Lutzenberger, Gruzelier, & Kaiser, 2003; Gevensleben, Rothenberger, Moll, & Heinrich, 2012; Moriyama et al., 2012). However, neurofeedback with EEG is limited with respect to spatial specificity, and thus of the brain regions which can be targeted. Neurofeedback with real-time fMRI offers the advantage of learning to control spatially localized brain activity within the range of millimeters (Birbaumer, Ruiz, & Sitaram, 2013; deCharms, 2007, 2008; Sulzer et al., 2013a; Weiskopf et al., 2004; Weiskopf et al., 2007). So far, few studies have employed this technically challenging method, however, the existing ones have demonstrated the feasibility of self-regulating activation in specific brain areas. Some studies have additionally shown that self-regulation leads to behavioral effects that are specific to the functional role of the targeted cortical area (Bray, Shimojo, & O’Doherty, 2007; Caria et al., 2007; deCharms et al., 2005; Rota et al., 2009; Scharnowski, Hutton, Josephs, Weiskopf, & Rees, 2012; Shibata, Watanabe, Sasaki, & Kawato, 2011; Weiskopf et al., 2003, 2004). Recently, studies have even demonstrated therapeutic effects of real-time fMRI neurofeedback training in chronic pain patients (deCharms et al., 2005), Parkinson's disease (Subramanian et al., 2011), tinnitus (Haller, Birbaumer, & Veit, 2010), and depression (Linden et al., 2012).
Most neurofeedback studies so far have trained participants to control activity within one region of interest (ROI). This was accomplished by either providing feedback from the ROI alone (Bray et al., 2007; Caria, Sitaram, Veit, Begliomini, & Birbaumer, 2010; Johnson et al., 2012; Johnston et al., 2011; Johnston, Boehm, Healy, Goebel, & Linden, 2010; Koush, Zvyagintsev, Dyck, Mathiak, & Mathiak, 2012; Mathiak et al., 2010; Subramanian et al., 2011; Weiskopf et al., 2003; Yoo et al., 2007; Yoo, Lee, O’Leary, Panych, & Jolesz, 2008), or by providing differential feedback between the ROI and either the contralateral homologue of the ROI (Chiew, LaConte, & Graham, 2012; Robineau et al., 2014) or some kind of background region (e.g. a reference slice) (Caria et al., 2007; deCharms et al., 2004; deCharms et al., 2005; Haller et al., 2010; Hamilton, Glover, Hsu, Johnson, & Gotlib, 2011; Hampson et al., 2011; Rota et al., 2009; Veit et al., 2012). Differential feedback has the advantage that global effects such as breathing, heart rate, unspecific changes due to arousal, and head movements are less likely to cause artifactual self-regulation. This is because these sources of artifacts affect the ROI as well as the background region, and are canceled out with differential feedback. In the present study, we extended the use of differential feedback by now using a second, functionally unrelated ROI instead of an unspecific background region, and by also including bidirectional control of the feedback signal (participants learned to voluntarily up- and down-regulate the feedback signal). Such bidirectional control also excludes that self-regulation can arise from unspecific effects related to task demands, such as attention or arousal. Any unspecific effects that are related to task demands will only allow to either increase or decrease the differential feedback signal, but will not allow bidirectional control.
The ROIs we trained were the supplementary motor area (SMA), which is involved in the control of movement (Grefkes, Eickhoff, Nowak, Dafotakis, & Fink, 2008; Koeneke, Lutz, Wustenberg, & Jancke, 2004; Nachev, Kennard, & Husain, 2008; Tanji, 2001), and the parahippocampal cortex (PHC), which is involved in memory encoding of visual scenes (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Gabrieli, Brewer, Desmond, & Glover, 1997; Stern et al., 1996; Turk-Browne, Yi, & Chun, 2006) and words (Fernandez, Brewer, Zhao, Glover, & Gabrieli, 1999; Otten, Quayle, Akram, Ditewig, & Rugg, 2006; Wagner et al., 1998). Because these two ROIs serve different brain functions, our paradigm involves the simultaneous training of two functionally distinct brain areas. We hypothesize that simultaneous differential training of the SMA and the PHC will cause behavioral effects that are linked to the functional role of each trained ROI. Specifically, we hypothesized that higher levels of SMA activity cause faster motor reaction times, and that higher levels of PHC activity cause improved memory. To test this hypothesis, we examined whether exercising voluntary control over SMA and PHC after neurofeedback training caused specific performance changes in a motor reaction time task and in a word memory task, respectively (Fig. 1).
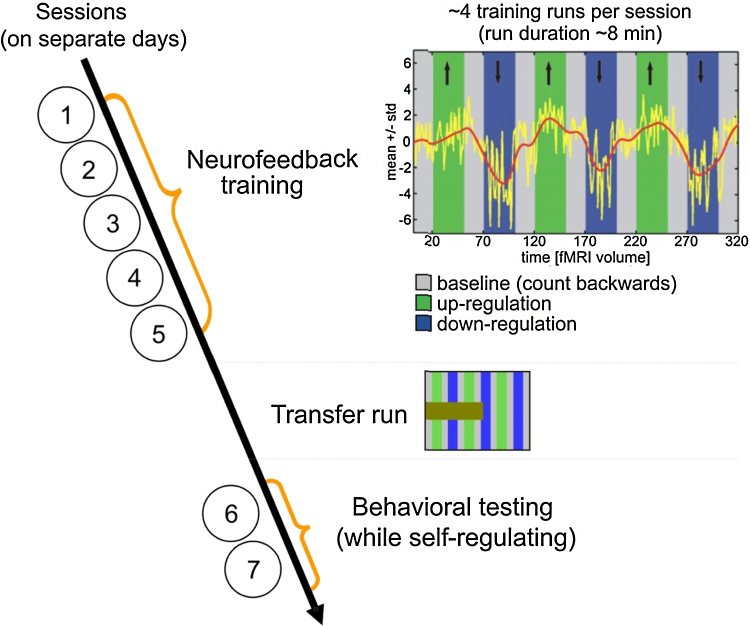
Fig. 1.
Experimental design. In order to learn simultaneous control over the level of ongoing activity in the SMA and in the PHC, participants underwent 12–22 runs of neurofeedback training spread over the course of 4-6 days, until they reached a pre-defined threshold of successful self-regulation. Each scanning session lasted ∼1 h. At the beginning of each neurofeedback training session, the ROIs were defined with functional localizers. Then, participants did on average 4 feedback runs of 8 min each per session. A feedback run was composed of 30 s baseline blocks (gray) interleaved with 45 s up- (green) and down-regulation (blue) blocks. The differential feedback signal was presented as a continuously updated yellow curve which was superimposed on the color-coded background illustrating the paradigm. For illustration purposes, a low-pass filtered (Gaussian FWHM = 25) version of the feedback signal is shown in red (this red curve and the black arrows were not presented during the experiment). After the training, participants tried self-regulation in the absence of feedback (transfer run), i.e. only the condition was indicated by a progress bar but not the feedback signal. Last, behavioral testing was performed in two separate scanning sessions on two separate days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2. Materials and methods
2.1. Functional MRI data acquisition
All experiments were performed on a 3 T Magnetom Trio scanner, using a standard transmit-receive head coil (Siemens Healthcare, Erlangen, Germany). Functional data were acquired with a single-shot gradient echo planar imaging sequence (matrix size: 64 × 64; resolution: 3.3 × 3.3 × 5 mm; 16 oblique transversal-coronal slices; slice thickness: 6 mm; slice gap: 1 mm; echo time TE: 35 ms; repetition time TR: 1500 ms; flip angle: 70°; receiver bandwidth: 2000 Hz/Px). For offline superposition of functional activations over anatomical details, we collected from each participant a high resolution T1-weighted structural scan of the whole brain (3D MDEFT; 1 mm isotropic resolution; matrix size: 256 × 240 mm; field of view: 256 × 240 mm; 176 sagittal partitions; echo time TE: 2.4 ms; repetition time TR: 7.92 ms; inversion time: 910 ms; flip angle: 15°; readout bandwidth: 195 Hz/pixel; spin tagging in the neck with flip angle 160° in order to avoid flow artifacts) (Deichmann et al., 2004).
The neurofeedback setup used Turbo-BrainVoyager (Brain Innovation, Maastricht, The Netherlands), custom real-time image export tools programmed in ICE VA25 (Siemens Healthcare) (Weiskopf et al., 2004), and custom scripts running on MATLAB (Mathworks Inc., Natick, MA, USA). The setup allowed participants to observe BOLD signal changes in specific brain regions with a delay of less than 1.5 s from the acquisition of the image. Head motion was corrected in real-time using Turbo-BrainVoyager.
2.2. Participants
Seven naïve adult volunteers (1 male, ages between 23 and 26 years, all right handed) with normal or corrected-to normal vision gave written informed consent to participate in the experiment. The experimental protocol was approved by the local ethics committee of the Faculty of Medicine, University of Tübingen, Germany. Before the experiment, they completed standardized tests assessing their spatial orientation ability (Stumpf & Fay, 1983), creative imagination (Barber & Wilson, 1978), and mood (Zerssen, 1976).
Participants received written instructions describing that they will be able to see their brain activity during the scanning and that they should raise or lower the feedback signal in accordance with the paradigm. The instructions included an explanation of the neurofeedback display (Fig. 1), and an explanation that they should not move and that they should breathe regularly. We also explained to the participants that the feedback was delayed by approximately 8 s (the hemodynamic delay plus the real-time analysis processing time). No background information about the differential feedback signal, or the anatomical areas was given. Initially, we also did not recommend any cognitive strategies for controlling the feedback signal. In order to facilitate learning, 6 out of the 7 participants received instructions that imagery of movements and spatial navigation might help to modulate the feedback curve after the third neurofeedback training session. Explicit strategies like imagery of fist clenching, skiing, navigating, and views of buildings or places were suggested as potential regulation strategies. Nevertheless, it was emphasized that participants should find an individual strategy that worked best for them.
After each scanning session, participants were asked to complete a written questionnaire and amongst other questions, describe how they tried to manipulate the feedback signal, how effective their strategy was, and how they rated the attentional demands.
2.3. Functional localizer runs
Each neurofeedback training session began with two functional localizer runs to delineate the ROIs from which the participants received feedback (Fig. 2). The first localizer run was used to define the SMA ROI. It consisted of five baseline blocks separated by three blocks of bimanual finger tapping. The second localizer run was used to define the PHC ROI. It consisted of three blocks of presentation of outdoor scenes alternating with three blocks of presentation of faces. All of these blocks were separated by baseline blocks. Block length was always 45 s. During baseline blocks, participants were instructed to count down from 100. Visual stimuli and instructions during localizer and feedback runs were displayed using a large circular projection screen at the rear of the scanner bore with a mirror positioned within the head-coil.
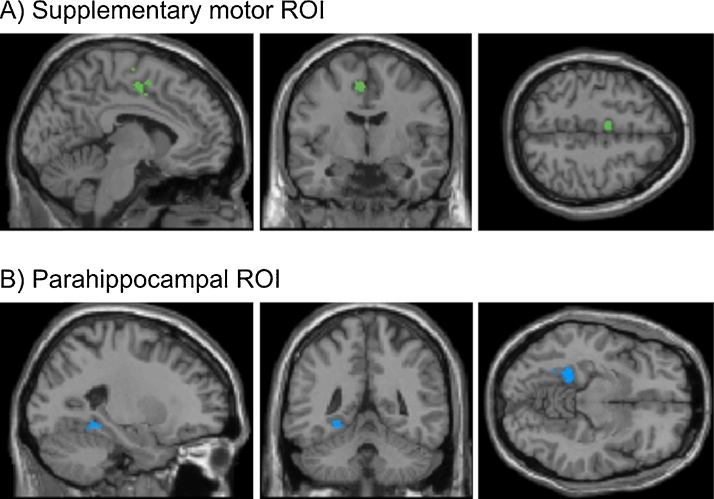
Fig. 2.
Illustration of the (A) SMA (green) and the (B) PHC ROIs (blue). In order to generate the average group ROIs, we first determined each participant's ROI based on all localizer runs, and then averaged over all participants. During the neurofeedback runs, the SMA ROI enclosed on average 26 voxels, and the PHC ROI 22 voxels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The SMA ROI for neurofeedback was restricted to those voxels in a rectangular region anterior of the paracentral sulcus and superior of the cingulate sulcus that exhibited a positive BOLD response to the finger tapping (p < 0.01, Bonferroni corrected for multiple comparisons). The PHC ROI for neurofeedback was restricted to those voxels in a rectangular region around the left parahippocampal gyrus that exhibited a greater BOLD response to houses in contrast to faces (p < 0.01, Bonferroni corrected for multiple comparisons).
2.4. Neurofeedback training
For each training session, participants performed on average 4 training runs of 8 min each. The training runs were composed of three 45 s up-regulation blocks and three 45 s down-regulation blocks, which were all interleaved with 30 s baseline blocks (Fig. 1). The blocks were color coded to indicate up-/or down-regulation blocks. During up-regulation blocks, the participants should increase the feedback signal. During down-regulation blocks, the participants should decrease the feedback curve. During baseline blocks, participants were instructed to mentally count backwards from 100. The order of up- and down-regulation blocks and the color assignment to up- and down-regulation was pseudo-randomized between participants, i.e. three volunteers were trained to up-regulate in regulation blocks 1,3, and 5, whereas four volunteers up-regulated in regulation blocks 2,4, and 6. Also the type of feedback signal was pseudo-randomized: Three volunteers were trained to control the SMA-PHC feedback signal, and four volunteers were trained to control the PHC-SMA feedback signal.
Participants were presented feedback via a continuously updated yellow curve which was superimposed on the color-coded background illustrating the condition (i.e. baseline, up-regulation, or down-regulation). The yellow curve represented the difference between the BOLD response of the SMA ROI and the PHC ROI, i.e. SMA-PHC for some and PHC-SMA for other participants. The differential feedback signal was normalized in relation to the mean and the standard deviation of the first baseline block. The maximum amplitude of the display was set to 10 standard deviations of the first baseline block.
After each run, self-regulation performance was quantified in SPM99 using a GLM consisting of 2 regressors indicating up- and down-regulation. Motion parameters (translation, rotation) were included as covariates to reduce the impact of residual motion artifacts. This GLM was not applied to the whole brain but only to the high-pass filtered (5.55 × 10−3 Hz) differential feedback signal. Both regressors were contrasted to determine signal differences between up- and down-regulation and the corresponding t-values were calculated. This analysis was carried out only to determine the further course of the experiment, and was not presented to the participants. Also, for the offline analysis, different statistical procedures were used. The neurofeedback training procedure was repeated until participants achieved a pre-defined criterion of successful self-regulation, i.e. when a t-value higher than 3.1 (which is equivalent to p < 0.001) was reached. Across participants, the training objectives were reached within 12–22 runs spread over the course of 4–6 days.
After successful neurofeedback training, participants performed self-regulation in the absence of feedback (transfer run). For this, the feedback curve was replaced by a large brown bar which provided only information about the timeline and the condition (i.e. baseline, up-regulation, or down-regulation) but not about the brain activations.
2.5. Behavioral test runs
A few days after the neurofeedback training, behavioral testing during self-regulation was performed in two separate scanning sessions spread over the course of two days, i.e. they were completely independent from the neurofeedback training and transfer sessions. During the behavioral test runs, the participants did not receive neurofeedback information.
For the reaction time test, participants had to perform one of two acoustically triggered bimanual finger sequences while self-regulating. A high (3000 Hz) or low pitch tone (1500 Hz) applied via headphones indicated the type of finger sequence. Both tones were presented for 100 ms at ∼80 dB Sound Pressure Level (SPL). When the high pitch tone was presented, participants had to press buttons of a MR compatible response box with their left index finger (D2), their right D2, their left middle finger (D3), and their right D3. For the low pitch tone the sequence of button presses was right D3, left D3, right D2, and left D2. In order to avoid anticipatory responses, the inter-stimulus interval of the acoustic cues was pseudo-randomized from 6 to 15 s. On average, the acoustic cues were presented every 10.5 s. During four test runs (same configuration as for the transfer runs), a total of 72 high and low pitch tones were presented during up- and down-regulation blocks. Motor sequences were recorded in real time with custom-made software (Muster5, MEG-Center, University of Tübingen, Germany). Before the test runs, subjects underwent 300 trials of pre-training outside of the scanner to become acquainted with the task. Reaction times were corrected for outliers by removing trials that were more than 2 standard deviations away from the mean. Only correct trials were analyzed. In order to assess reaction time differences between the up- and down-regulation blocks, a paired t-test was applied on the group level (two-tailed; statistical significance threshold of p < 0.05).
For the word memory task, participants had to process words while self-regulating. Due to technical problems, only six out of the seven participants performed this task. These words were presented in capital letters every six seconds above the feedback display. They were presented for 1.5 s and encompassed approximately 5° of visual angle. During baseline blocks, no words were presented. To ensure that the participants attended to the words, they were asked to detect and indicate randomly interspersed pseudo words by pressing a button. Participants were not instructed to memorize the words, and they did not know that their memory for these words was later on assessed by an unexpected word recognition test. During 3 test runs (same configuration as for the transfer runs), a total of 123 words (3 × 35 words and 3 × 6 pseudo words) were presented. They were nouns or verbs out of three semantic categories: space, movement, and neutral (consisting of words related to animals or food). Between categories, all words were balanced for frequency, type, category, and length according to the Mannheim Corpus of German language (Institut fuer Deutsche Sprache, Mannheim, Germany).
Approximately 20 min after the last imaging run, participants were administered an unexpected recognition test. For this, they were presented with a written list of words, containing all the presented words and the same number of new words (which were semantically and linguistically balanced as the test words). Participants had to indicate if a given word had been presented during any of the 3 preceding behavioral test scans by tagging ‘sicher’ (German for ‘sure’) if they were absolutely sure of having seen the word, ‘unsicher’ (German for ‘unsure’) in case it appeared familiar, and ‘neu’ (German for ‘new’) otherwise. A word that has been presented during the behavioral test scans was classified as remembered when the participant was absolutely sure or believed to have seen the word during the scanning. A word that has been presented during the recognition test scans was classified as forgotten when the participant labeled it as new. In order to assess word memory differences between the up- and down-regulation blocks, recognition performance was compared on the group level using a sign rank test (statistical significance threshold of p < 0.05).
2.6. Offline analysis
2.6.1. Initial offline data preprocessing
Offline data analysis was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, Queen Square, London, UK; http://www.fil.ion.ucl.ac.uk/) and BrainVoyager QX (Brain Innovation). The first 10 volumes of each run were excluded from statistical analysis to allow for T1-related equilibration. The remaining images were corrected for slice time acquisition differences, realigned to the first scan of each run, coregistered to the structural scan, normalized to MNI space, and smoothed with an isotropic Gaussian kernel with 4 mm full-width-at-half-maximum (FWHM).
2.6.2. Offline ROI analysis
In order to assess the neurofeedback training success, we specified GLMs with regressors for the optimal differential feedback signal, the optimal SMA time course, or the optimal PHC time course. For example, if the participant was trained to up-regulate the differential feedback signal SMA-PHC in regulation blocks 2, 4, and 6 (and consequently to down-regulate in regulation blocks 1, 3, and 5), then the regressors for the optimal differential feedback signal as well as the optimal SMA time course was set to 1 in regulation blocks 2, 4, and 6, and to −1 in regulation blocks 1, 3, and 5. The optimal PHC time course regressor in this example was set to –1 in regulation blocks 2, 4, and 6, and to 1 in regulation blocks 1, 3, and 5. The regressors were modeled as boxcar functions convolved with the canonical hemodynamic response function (HRF) in SPM8. The beta parameter estimates were computed separately for each ROI time course of each run. Per GLM, only a single regressor was used, i.e. the optimal differential feedback signal regressor for the differential feedback time course, the optimal SMA time course regressor for the SMA time course, and the optimal PHC time course regressor for the PHC time course.
Because the number of completed training runs varied slightly across participants, i.e. participants completed 19, 20, 17, 17, 12, 19, or 22 training runs, the beta parameter estimates for each participant were grouped and averaged into 12 bins (=minimum number of training runs that all participants completed) in a nearest neighbor fashion. To assess the neurofeedback learning effect, linear regressions of the mean beta parameter estimates over training runs were calculated for the differential feedback signal, for the SMA time course, and for the PHC time course. In addition, t-tests were calculated to examine regulation success in the training runs, in the transfer runs, and in the behavioral test runs (two-tailed; statistical significance threshold of p < 0.05). The same analyses were carried out separately for participants that were trained to control SMA-PHC and for participants that were trained to control PHC-SMA.
In order to investigate the relative contribution of the SMA and the PHC to up- vs. down-regulation of the differential feedback signal, we separately computed the percentage of signal change in the SMA and the PHC of the last 5 training runs (these runs showed the best control over the feedback signal). This was done separately for SMAup/PHCdown blocks, and for SMAdown/PHCup blocks. The time courses were normalized so that the percentage of signal change during baseline activity corresponded to 0%, i.e. the average baseline signal change was subtracted from each time point during the regulation blocks.
2.6.3. Whole brain analyses
In first level analysis, we specified GLMs with regressors for the up-regulation condition, and covariates derived from head movement parameters to capture residual motion artifacts. The regressors were modeled as boxcar functions convolved with the canonical hemodynamic response function (HRF) in SPM8. In second level, we calculated fixed-effect group analyses contrasting self-regulation vs. baseline, SMAup/PHCdown blocks vs. baseline, SMAdown/PHCup blocks vs. baseline, and SMAup/PHCdown blocks vs. SMAdown/PHCup blocks of the last training run. For the comparisons with baseline, a positive contrast was applied to reveal brain activations, and a negative contrast to reveal deactivations. Statistical parametric maps were thresholded at p < 0.05 corrected for multiple comparisons using the family wise error rate (FWE). The averaged ROIs were used to perform small volume correction of the whole brain analyses (Worsley et al., 1996). Random-effects analyses did not reveal significant effects due to the low number of participants (except for significant changes in the ROIs after small volume correction based on the average ROI). The results of the whole brain analyses therefore cannot be generalized beyond the study sample.
2.6.4. Exploration of connectivity changes using psychophysiological interaction (PPI) analysis
To explore connectivity changes due to learned self-regulation, we conducted a psychophysiological interactions analysis (PPI, Friston et al., 1997) between different brain areas and activity in the ROIs. For the PPI analysis, we specified general linear models (GLMs) with regressors for the respective ROI time course, for the experimental conditions (i.e. a boxcar function representing up- and down-regulation and baseline blocks convolved with the canonical hemodynamic response function in SPM8), and for the interaction between the two. This was done separately for the SMA ROI and the PHC ROI time courses. To reveal areas of the brain whose connectivity to the respective ROI changed depending on self-regulation, we first applied a positive contrast to parameters estimated for the interaction term, and then calculated a voxelwise 1-sample t-test of the interaction term contrast images of each participant's last training run. Statistical parametric maps were thresholded at p < 0.05 corrected for multiple comparisons using FWE.
In addition to exploring psychophysiological interactions across the whole brain, we specifically assessed the interaction between the SMA ROI with the PHC ROI. For this, we extracted the mean PPI parameter estimate at the location overlapping with the SMA ROI from the whole brain PPI analysis that was based on the PHC ROI time course. Vice versa, to reveal the interaction between the PHC ROI with the SMA ROI, we extracted the mean PPI parameter estimate at the PHC ROI location from the whole brain PPI analysis that was based on the SMA ROI time course. This was done for each participant and for each neurofeedback training run. In order to assess interaction changes across training runs, we calculated a linear regression of the group average over training runs (statistical significance threshold of p < 0.05).
2.6.5. Correlation between reaction times and ROI activity
To investigate how voluntary self-regulation influenced reaction times, we correlated the activity in the ROIs at the time of the acoustic motor response trigger with reaction times of the respective trial (i.e. the time it took until the finger sequence was initiated). For this, we calculated the mean percentage of signal change in the regulation blocks compared to the baseline blocks for each run, and we z-transformed the reaction times to allow for between-subject comparison. Finally, we performed a linear regression analysis correlating the activity at the SMA ROI as well as the PHC ROI at the time of the acoustic cue and the reaction times. The factor subjects were included as a random effects variable of no interest to account for inter-subject variance. The same correlation analysis was carried out for the duration of the finger tapping sequence, i.e. for the time it took to perform the complete finger tapping sequence.
In addition, we plotted the percentage of signal change time courses of the ROIs from the time of the auditory cue to the execution of the motor response. This was done separately for up- and down-regulation blocks. To compare each ROI's activity in the up-regulation vs. the down-regulation blocks at the time of the auditory cue, we calculated paired t-tests (two-tailed; statistical significance threshold of p < 0.05).
3. Results
3.1. Learning voluntary control of SMA and PHC
Each participant completed at least 12 neurofeedback training runs spread over the course of 4–6 days. Over the course of this training, participants successfully learned to control the differential feedback signal. Specifically, participants showed a significant increase in beta parameter estimates for the differential feedback signal associated with training (Fig. 3; differential signal linear regression: r2 = 0.83, F(1,10) = 47.39, p < 0.01). An exemplary time course of successful regulation of the differential feedback signal is shown in Fig. 1. Participants accomplished this by learning to self-regulate both the SMA (Fig. 3; SMA linear regression: r2 = 0.56, F(1,10) = 12.58, p < 0.01) as well as the PHC (Fig. 3; PHC linear regression: r2 = 0.71, F(1,10) = 23.87, p < 0.01) components of the differential feedback signal.
Fig. 3.
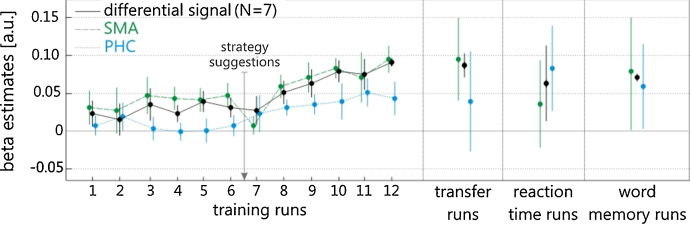
Neurofeedback learning performance. Self-regulation performance was measured as beta parameter estimates, which indicate how close the measured signal was following the regulation task. The 7 participants showed an increase in differential feedback signal control with training. This increase is mediated by voluntarily controlling both components of the differential feedback signal, i.e. the SMA and the PHC ROIs. Voluntary control was maintained during transfer runs and during the behavioral test runs. After the ∼6th training run, specific regulation strategies related to motor imagery and spatial navigation were suggested to the participants. Error bars represent one standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
This learned ability to control the differential feedback signal was subsequently maintained in the absence of neurofeedback. This was shown in transfer runs, where we tested the ability of trained participants to regulate the differential feedback signal in accordance with the paradigm, but this time in the absence of neurofeedback (Fig. 3, transfer run column; differential signal 1-sample t-test: t(6) = 8.41, p < 0.01; see Fig. 1 for an illustration of the experimental display during transfer runs). During the transfer runs, regulation of the differential feedback signal was accomplished by mainly controlling activity in the SMA (Fig. 3, transfer run column; SMA 1-sample t-test: t(6) = 4.27, p < 0.01; PHC 1-sample t-test: t(6) = 1.36, p = 0.22).
Because we pseudo-randomized the type of feedback signal (i.e. three volunteers were trained to control the SMA-PHC feedback signal, and four volunteers were trained to control the PHC-SMA feedback signal), we also investigated performance separately for these respective sub-groups. Learning control of the differential feedback signal did not depend on whether the participants received SMA–PHC or PHC–SMA feedback (see Supplemental Fig. S1).
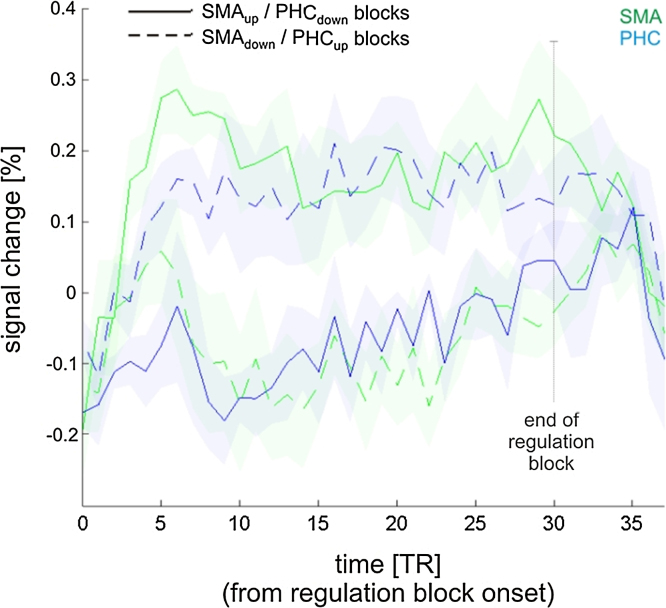
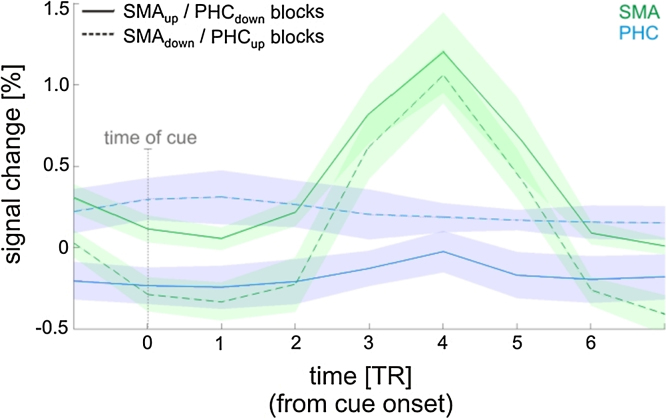
Both up- and down-regulation of each ROI contributed to successful self-regulation. During SMAup/PHCdown blocks, activity in the SMA was increased and at the same time activity in the PHC was decreased (Fig. 4). Likewise, during SMAdown/PHCup blocks, activity in the SMA was decreased and activity in the PHC was increased. Please note that the distinction into SMAup/PHCdown and SMAdown/PHCup blocks was done post-hoc, i.e. the participants were not aware of this distinction and were only instructed to up- and down-regulate the feedback signal (see Section 2 for details).
Fig. 4.
ROI time courses separately for SMAup/PHCdown and SMAdown/PHCup blocks of the last training runs. SMA activity (green) was increased during SMAup/PHCdown blocks (solid lines), and decreased during SMAdown/PHCup blocks (dashed lines). In contrast, PHC activity (blue) was decreased during SMAup/PHCdown blocks, and increased during SMAdown/PHCup blocks. This illustrates that successful self-regulation was accomplished by up- and down-regulating the respective ROI. Shaded areas represent one standard error of the mean. The time courses were normalized so that the percentage of signal change during baseline activity corresponded to 0%.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Neural processes underlying control of the differential feedback signal
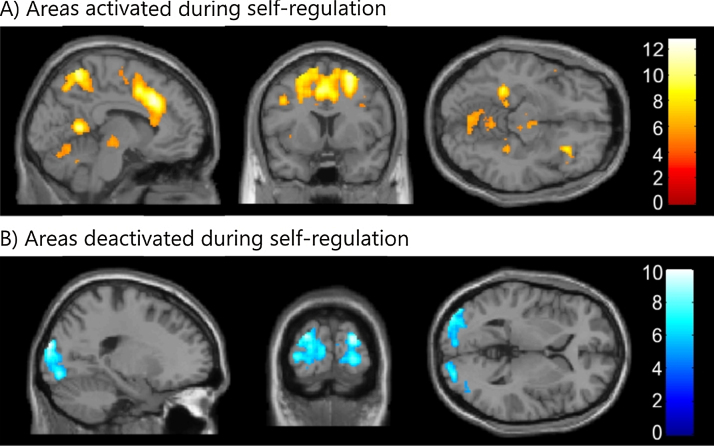
Whole-brain group-level analyses revealed specific patterns of brain activity during self-regulation blocks, i.e. SMAup/PHCdown and SMAdown/PHCup blocks combined. Activation increases during self-regulation blocks included the SMA and the PHC ROIs, the middle cingulate cortex bilaterally, the left superior parietal lobe, the right superior frontal gyrus, the precuneus bilaterally, the cerebellum bilaterally, the inferior parietal cortex bilaterally, the right hippocampus, and the left putamen (Fig. 5A; Table 1). Activation decreased in the superior and ventral visual cortex bilaterally (Fig. 5B; Table 1).
Fig. 5.
Whole brain analyses. Shown are brain activation maps for (A) increased activity during self-regulation blocks, and (B) deactivations during self-regulation blocks. Activation increases during self-regulation blocks included the SMA and the PHC ROIs, the middle cingulate cortex bilaterally, the left superior parietal lobe, the right superior frontal gyrus, the precuneus bilaterally, the cerebellum bilaterally, the inferior parietal cortex bilaterally, the right hippocampus, and the left putamen. Activation decreased in the superior and ventral visual cortex bilaterally. The figures show contrast maps thresholded at p < 0.05 (corrected for multiple comparison using FWE) on the MNI template brain. For details, see Table 1.
Table 1.
Areas related to self-regulation (group whole brain analysis).
| Areas de-/activated during self-regulation blocks (i.e. SMAup/PHCdown and SMAdown/PHCup blocks) | |||||
|---|---|---|---|---|---|
| Anatomical label | t-Value | De-/activation [−/+] | MNI coordinates |
||
| x | y | z | |||
| Bilateral middle cingulate cortex | 12.77 | + | −2/8 | 25 | 32 |
| Left superior parietal lobe | 12.42 | + | −18 | −70 | 42 |
| Right superior frontal gyrus | 12.40 | + | 26 | 3 | 58 |
| Bilateral precuneus | 10.86 | + | −6/14 | −55 | 10 |
| Left parahippocampal gyrus | 10.47 | + | −30 | −40 | −10 |
| Bilateral cerebellum (lobule VI) | 8.67 | + | −30/34 | −64 | −24 |
| Bilateral inferior parietal cortex | 8.62 | + | −48/40 | −71 | 28 |
| Bilateral SMA | 8.11 | + | −2/10 | −12 | 64 |
| Right hippocampus | 7.61 | + | 30 | −36 | −14 |
| Left putamen | 6.14 | + | −24 | −2 | 12 |
| Bilateral superior occipital cortex | 9.89 | – | −22/24 | −94 | 20 |
| Bilateral ventral occipital cortex | 8.98 | – | −22/28 | −86 | −9 |
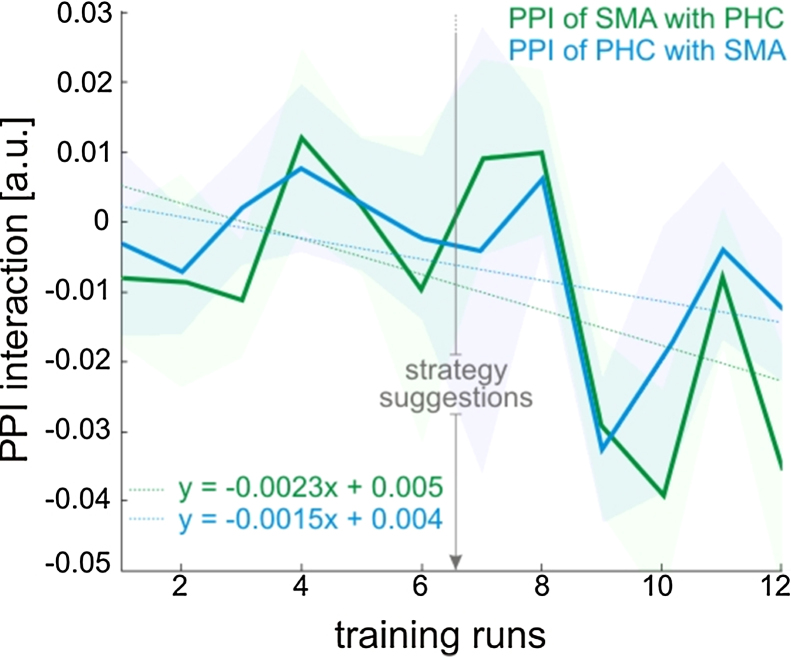
To provide further insight into the neural processes underlying control of the differential feedback signal, we explored psychophysiological interactions (PPI) with activity in the SMA ROI as well as the PHC ROI (Friston et al., 1997). The explorative whole brain PPI analysis revealed no region whose connectivity to the SMA or the PHC changed significantly. However, when inspecting the ROIs themselves, we found that during self-regulation, activity in the SMA ROI was negatively coupled to activity in the PHC ROI (Fig. 6; PPI of SMA with PHC 1-sample t-test of the last training run: t(6) = −2.47, p = 0.04). Naturally, activity in the PHC ROI was also negatively coupled to activity in the SMA ROI, although this coupling did not reach significance (Fig. 6; PPI of PHC with SMA 1-sample t-test of the last training run: t(6) = −1.13, p = 0.30). There was a trend towards an increase in negative coupling between the SMA and the PHC across the training sessions (Fig. 6; PPI of SMA with PHC linear regression: r2 = 0.217, F(1,10) = 2.77, p = 0.13; PPI of PHC with SMA linear regression: r2 = 0.232, F(1,10) = 3.01, p = 0.11).
Fig. 6.
Psychophysical interaction between the SMA and the PHC across neurofeedback training. With training, there was a trend towards an increase in negative coupling between the SMA and the PHC across the training sessions (Fig. 6; PPI of SMA with PHC linear regression: r2 = 0.217, F(1,10) = 2.77, p = 0.13; PPI of PHC with SMA linear regression: r2 = 0.232, F(1,10) = 3.01, p = 0.11). Shaded areas represent one standard error of the mean.
3.3. Cognitive processes underlying control of the differential feedback signal
As part of the debriefing after the neurofeedback training sessions, participants were asked how they attempted to regulate the feedback signal. Initially, the participants described their imagery as, for example, ‘having positive (for up-regulation) or negative (for down-regulation) emotions’, ‘thinking of flying birds (for up-regulation) or diving fish (for down-regulation), or they tried to control the feedback by looking at the location where they wanted the feedback signal to be. However, these cognitive strategies did not work for all but one participant, and successful self-regulation was achieved only after we recommended potential regulation strategies that were related to the functional role of the SMA and PHC (Fig. 3; strategy suggestions; see Section 2 for details). For all but one participant, after the second training day, we suggested the use of motor and spatial navigation imagery, but we did not specify when these regulation strategies might be most effective. Following our recommendations, some participants increased activity in the SMA by, for example, imagining ‘playing the piano’, ‘doing sports’, or ‘dancing’. To increase activity in the PHC, some participants described their imagery as ‘navigating home’, ‘driving home’, or ‘walking through the apartment’.
However, we emphasized that participants had to find their own best strategy, and some of their cognitive strategies did not fit into the category of motor-related or navigation-related imagery. For example, one participant, who received SMA-PHC feedback, ‘imagined numbers’ in order to up-regulate the feedback signal and ‘relaxed’ or ‘imagined dark colors’ in order to down-regulate the feedback signal. This participant nevertheless successfully learned to control the differential feedback signal (differential feedback signal 1-sample t-test across all training runs: t(19) = 3.36, p < 0.01). For this participant, the PHC ROI contributed more to successful self-regulation (PHC 1-sample t-test across all training runs: t(19) = 2.92, p < 0.01) than did the SMA ROI (SMA 1-sample t-test across all training runs: t(19) = 1.79, p < 0.08).
One participant even successfully controlled the differential feedback signal without receiving recommendations for potential regulation strategies (differential feedback signal 1-sample t-test across all training runs: t(11) = 8.98, p < 0.01). This participant received PHC-SMA feedback and thought about ‘the effects of radiation therapy’ or ‘imagined the layout of an x-ray machine’ in order to increase the feedback signal. In order to decrease the feedback signal this participant ‘imagined their work environment/apartment’. Given the functional role of the PHC, especially the latter strategy should increase rather than decrease the PHC activity and consequently not lead to a decreasing differential feedback signal. Indeed, we found that this participant learned to regulate the differential feedback signal by regulating the SMA (SMA 1-sample t-test across all training runs: t(11) = 10.57, p < 0.01), but that she did not learn to regulate the PHC (PHC 1-sample t-test across all training runs: t(11) = 1.14, p = 0.28).
In order to elucidate the psychological underpinnings of successful self-regulation, we also assessed the participants’ ability in spatial orientation (Stumpf & Fay, 1983), creative imagination (Barber & Wilson, 1978), and mood (Zerssen, 1976). However, none of these psychological questionnaires was predictive with respect to regulation success (all ps > 0.05).
3.4. Behavioral effects of self-regulation: The reaction time task
During the behavioral test session, participants showed significant control over the differential feedback signal (Fig. 3, SMA test run column; differential signal 1-sample t-test: t(6) = 2.97, p = 0.02). As anticipated, due to the simultaneous behavioral experiment, self-regulation of the differential feedback signal was somewhat less successful than during the training and transfer runs, but it still demonstrated statistically significant differences from baseline. Likewise, self-regulation of the SMA ROI alone was no longer evident over the behavioral test session because it was masked by SMA activity related to overt finger movements (Fig. 3, SMA test run column; SMA 1-sample t-test: t(6) = 1.60, p = 0.16). However, at the time of the auditory cue, which triggered the motor response, SMA activity was significantly higher during the SMAup/PHCdown blocks than during the SMAdown/PHCup blocks (Fig. 7; paired t-test: t(6) = 3.29, p = 0.02). The opposite pattern was found for the PHC (t-test: t(6) = –2.50, p = 0.04).
Fig. 7.
ROI time courses at the time of the auditory cue presentation. During the reaction time task session, participants achieved a significant increase in SMA activity (green) in SMAup/PHCdown blocks compared to SMAdown/PHCup blocks at the time of the cue. The opposite can be found for the PHC (blue), which indicates that both ROIs were successfully modulated during this session. Also, the effect of the overt motor response is visible as increasing SMA activity a few time points after the auditory cue. Shaded areas represent one standard error of the mean.
When collapsing across all trials, the increase in SMA activity (and the decrease in PHC activity) that the participants achieved during the test session was not associated with a significant decrease in reaction times (Fig. 8A paired t-test: t(6) = −0.56, p = 0.60).
Fig. 8.
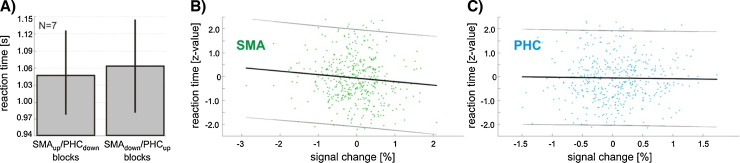
Reaction times. (A) There was no significant difference in reaction times between SMAup/PHCdown blocks compared to SMAdown/PHCup blocks. Error bars represent one standard error of the mean. (B) During the reaction time session, there was a significant negative correlation between self-regulated activity in the SMA at the time of the auditory cue presentation and reaction times. (C) Such a correlation was not found for the PHC. Please note the different scaling of the x-axes. Gray lines indicate 95% confidence intervals.
There was, however, a significant negative correlation between the self-regulated activity in the SMA and the reaction times across trials over the behavioral test session, i.e. the more the participants increased/decreased activity in the SMA, the faster/slower they responded (Fig. 8B; SMA linear regression: r2 = −0.15, F(1,453) = 4.21, p = 0.04). Reaction times did not correlate with activity in the PHC ROI (Fig. 8C; PHC linear regression: r2 = −0.03, F(1,453) = 0.14, p = 0.71). This correlation was specific to the onset of the movement and was not evident for the duration of the movement, i.e. the time it took to perform the finger sequence (SMA linear regression: r2 = 0.04, F(1,453) = 0.29, p = 0.59; PHC linear regression: r2 = −0.04, F(1,453) = 0.23, p = 0.64).
3.5. Behavioral effects of self-regulation: The memory task
During the behavioral test session, participants showed significant control over the differential feedback signal (Fig. 3, PHC test run column; differential signal 1-sample t-test: t(6) = 3.97, p < 0.01). Again, due to the simultaneous behavioral experiment, self-regulation of the differential feedback signal was somewhat less successful than during the training and transfer runs, but it still demonstrated statistically significant differences from baseline. Self-regulation during the word memory task was evident in both the SMA ROI (Fig. 3, PHC test run column; SMA 1-sample t-test: t(6) = 2.75, p = 0.03) as well as in the PHC ROI (Fig. 3, PHC test run column; PHC 1-sample t-test: t(6) = 2.59, p = 0.04).
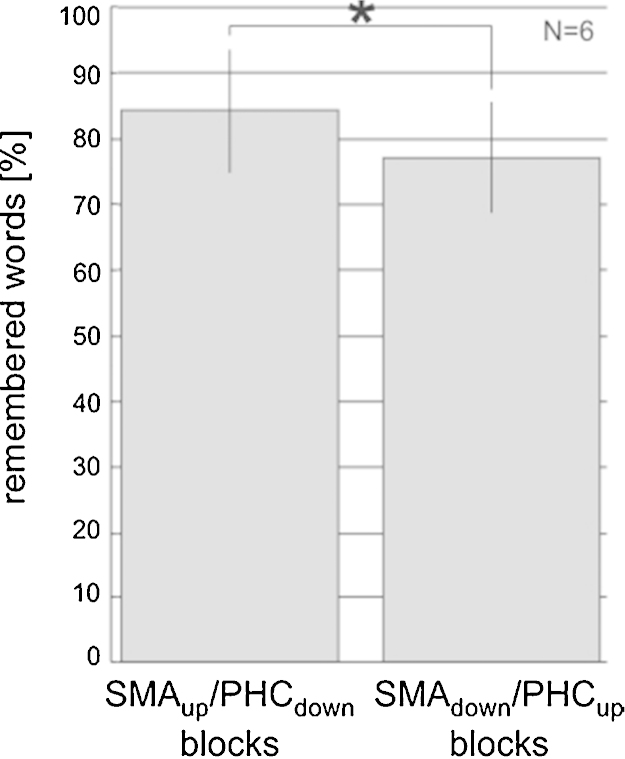
The increase in PHC activity (or decrease in SMA activity) that the participants achieved during the test session was associated with a significant decrease in memory for words, and this in all participants (Fig. 9; Wilcoxon sign rank test: p = 0.03).
Fig. 9.
Memory for words. Participants remembered significantly more words that had been presented during SMAup/PHCdown blocks, compared to words that had been presented during SMAdown/PHCup blocks. Error bars represent one standard error of the mean.
4. Discussion
Using differential real-time fMRI neurofeedback, we demonstrated that participants could learn to simultaneously control activity in the SMA and the PHC (Fig. 3). The control over the feedback signal was subsequently maintained in transfer runs where participants no longer received neurofeedback information. When participants voluntarily regulated activity in these regions, significant changes in motor reaction times and memory performance were observed, which were specific to the differential self-regulation.
4.1. Neurofeedback learning
Through neurofeedback training, the participants in our study achieved control over the differential feedback signal (Fig. 3). The control over the feedback signal was subsequently maintained in transfer runs where participants no longer received neurofeedback information, and in behavioral test runs where participants had to perform additional behavioral tests while self-regulating.
How was such control achieved by the participants? In order to elucidate the neural underpinnings of successful self-regulation, we analyzed how well the ROI activity changes followed the requirements of the experimental paradigm. We found that participants simultaneously regulated activity in the SMA as well as in the PHC, i.e. both ROIs contributed to successful self-regulation (Fig. 3; SMA in green, PHC in blue). However, this could have been achieved without true bidirectional control of each ROI, for example, by up-regulating the SMA during SMAup/PHCdown blocks (while the PHC remains unchanged) and up-regulating the PHC during SMAdown/PHCup blocks (while the SMA remains unchanged). We therefore analyzed the ROI time courses and found that the ROIs were not only up-regulated, but also down-regulated (Fig. 4). For example, when a participant received SMA-PHC differential feedback, then activity in the SMA increased during up-regulation blocks and decreased during down-regulation blocks, and activity in the PHC decreased during up-regulation blocks and increased during down-regulation blocks. This negative coupling between the SMA and the PHC was also evident in the PPI analysis, which revealed connectivity changes between both regions that changed with training (Fig. 6). Such bidirectional control excludes that self-regulation and the resulting behavioral consequences are due to unspecific effects such as attention or arousal, providing a strong within-subject control.
To shed further light on the neural substrate of neurofeedback learning, we applied whole brain analyses to reveal brain activations extending beyond the SMA and PHC ROIs. Similar to previous real-time fMRI neurofeedback studies, we found that self-regulation resulted in widespread brain activations (e.g. Chiew et al., 2012; Haller et al., 2013; Rota, Handjaras, Sitaram, Birbaumer, & Dogil, 2011; Subramanian et al., 2011; Sulzer et al., 2013b; Veit et al., 2012; Zotev et al., 2011). These activations included the SMA and PHC ROIs, attention-related parietal areas, cingulate areas which might be involved in reward-based learning, and areas related to skill learning such as the putamen (Fig. 5; Table 1). Especially the involvement of motor circuits and the basal ganglia are interesting, because they are consistent with a recently proposed theory according to which neurofeedback learning is akin to skill learning (Birbaumer et al., 2013). When considering only SMAup/PHCdown blocks, motor areas but not parahippocampal areas were activated (see Supplemental Fig. S2; Supplemental Table S1). Likewise, during SMAdown/PHCup blocks, the SMA was no longer activated (see Supplemental Fig. S2; Supplemental Table S1). This reflects the fact that up-regulation of the ROIs was more pronounced than their down-regulation (see also Fig. 3). Nevertheless, at a lower statistical threshold, brain activation maps also indicate deactivations of the respective ROI, i.e. of the PHC during SMAup/PHCdown blocks and of the SMA during SMAdown/PHCup blocks (not shown). Surprisingly, we found consistent deactivations in the visual cortex even though there was no difference in the visual display between regulation and baseline blocks, and despite the use of imagery as a cognitive control strategy during regulation blocks (Guillot et al., 2009; Kosslyn, Ganis, & Thompson, 2001; Slotnick, Thompson, & Kosslyn, 2005; Stokes, Thompson, Cusack, & Duncan, 2009).
In order to elucidate the cognitive processes underlying successful self-regulation, we debriefed the participants after the neurofeedback training sessions. As part of the debriefing, participants described the contents of their imagery. They initially tried various strategies such as imagining positive/negative emotions or looking at the location on the screen where they wanted the feedback signal to be. These strategies were not suitable for controlling the feedback signal. For most participants, learning was evident only after we suggested the use of potential regulation strategies that were related to the functional roles of the SMA and the PHC, e.g. imagining dancing or navigating home, respectively (Fig. 3; strategy suggestions). This finding suggests that the ability to control the feedback signal is due to a feedback-guided search for a cognitive control strategy.
On closer inspection, however, explicit cognitive control strategies cannot entirely explain the neurofeedback learning in our study. The specific cognitive strategies that the participants used potentially only explain the activity increase in the respective ROIs, but they are not linked to activity decreases of the other ROI. For example, while it is known that motor imagery increases activity in the SMA (e.g. Guillot et al., 2009), it is unclear how such a strategy could at the same time decrease activity in the PHC. Likewise, while it is known that spatial navigation imagery increases activity in the PHC (e.g. O’Craven & Kanwisher, 2000), it is unclear how such a cognitive strategy could at the same time decrease SMA activity. Further, even after we suggested control strategies related to motor and navigation imagery, some participants found other unrelated strategies more effective. One participant even learned to control the feedback signal without any strategy suggestions.
The fact that we initially did not suggest control strategies allowed us to shed new light on the potential learning mechanisms involved in neurofeedback training. We found that cognitive control strategies strongly facilitate neurofeedback learning. However, a model of neurofeedback learning that rests exclusively on explicit cognitive processing cannot entirely explain our results, e.g. it cannot explain how bidirectional control of the ROIs was achieved. Other feedback studies using electroencephalography (EEG) or physiological signals like heart-rate found evidence for the involvement of explicit cognitive control strategies, but also of more implicit operant conditioning based on reinforcement provided by the feedback (Dunn, Gillig, Ponsor, Weil, & Utz, 1986; Kotchoubey, Kubler, Strehl, Flor, & Birbaumer, 2002; Lacroix & Roberts, 1978; Neumann, Kubler, Kaiser, Hinterberger, & Birbaumer, 2003; Roberts, Birbaumer, Rockstroh, Lutzenberger, & Elbert, 1989; Roberts, Williams, Marlin, Farrell, & Imiolo, 1984; Schober & Lacroix, 1986; Siniatchkin, Kropp, & Gerber, 2000; Utz, 1987, 1994). Operant conditioning based neurofeedback has even been used to train volitional control of the activity of single neurons in animals (Fetz, 1969, 2007; Olds, 1965). However, because of the differences between these methods and real-time fMRI-based neurofeedback, it remains unclear if these findings can be generalized. Despite the lack of understanding of how real-time fMRI-based neurofeedback learning is accomplished, participants are usually given explicit control strategies. Only few studies in the field have used an operant conditioning approach (Bray et al., 2007), or have successfully trained participants without having suggested cognitive control strategies (Shibata et al., 2011). Our results thus indicate that suggesting explicit control strategies may initially facilitate learning, but that they are not necessary (Birbaumer et al., 2013; Shibata et al., 2011). Our results do not allow us to draw inferences about the need for such strategies during later stages of neurofeedback learning, or during the maintenance of learned self-regulation.
4.2. Behavioral effects of SMA and PHC control
After the neurofeedback training, we tested if exercising voluntary control over SMA and PHC led to ROI-specific performance changes. To test for behavioral consequences of regulating activity in the SMA, we asked the participants to perform a complex motor reaction time task. This task involved the planning and initiation of movements that require memorized, sequential, bilateral coordination; all of which are processes that are mediated by the SMA (Koeneke et al., 2004; Nachev et al., 2008; Tanji, 2001). The SMA has dense cortico-cortical connections with primary motor cortex (Johansen-Berg et al., 2004; Luppino, Matelli, Camarda, & Rizzolatti, 1993; Tokuno & Nambu, 2000), and it has been shown that activity in the SMA can modulate the cortical excitability of primary motor cortex (Arai, Lu, Ugawa, & Ziemann, 2012; Arai et al., 2011; Grefkes et al., 2008; Hamada et al., 2009; Matsumoto et al., 2007; Matsunaga et al., 2005; Shirota et al., 2012). We therefore hypothesized that voluntarily increasing activity in the SMA will lead to reduced reaction times. Our results partly confirmed this hypothesis. While we found no significant difference in reaction times between SMAup/PHCdown blocks compared to SMAdown/PHCup blocks, we found a significant negative correlation between self-regulated activity in the SMA and reaction times (Fig. 8). The correlation we found was specific to the initiation of the movement, but self-regulation of SMA activity did not affect the execution (i.e. the time it took to perform the finger sequence). This might be due to the fact that participants practiced the motor sequence extensively, and that during the production of highly practiced sequences, the SMA (and pre-SMA, which was partly included in our ROI) is especially important in the retrieval and initiation of sequences (Johansen-Berg et al., 2004; Kennerley, Sakai, & Rushworth, 2004; Tanji & Shima, 1994; Wiestler & Diedrichsen, 2013; Yanaka, Saito, Uchiyama, & Sadato, 2010).
Previous studies that successfully trained participants control over SMA activity did not test for behavioral effects of self-regulation (Hampson et al., 2011; Johnson et al., 2012). Amongst the studies that trained participants control over primary motor cortex (Bray et al., 2007; Chiew et al., 2012; deCharms et al., 2004; Yoo et al., 2008) only two reported behavioral effects. One study found that increased primary motor cortex activity caused a decrease in reaction times (Bray et al., 2007), and the other study did not find changes in reaction times after compared to before neurofeedback training of the primary motor cortex (Chiew et al., 2012). Instead of measuring reaction time differences between pre- vs. post-training of primary motor cortex, we measured reaction times while participants were actively up- and down-regulating activity in the SMA, i.e. participants were applying their newly learned self-regulation skill.
To test for behavioral consequences of regulating activity in the PHC, we presented words while participants regulated activity in their PHC. Afterwards, memory for these words was tested in an unexpected word memory test. The PHC is the principal neocortical input pathway to the hippocampus and is involved in memory formation. It has been shown that greater stimulus-evoked activity in the PHC correlates with better memory for scenes (Brewer et al., 1998; Gabrieli et al., 1997; Stern et al., 1996) and for words (Fernandez et al., 1999). Even more important for the present study, it has been shown that pre-stimulus PHC activity predicts memory for scenes (Turk-Browne et al., 2006) and words (Otten et al., 2006; Wagner et al., 1998), i.e. greater pre-stimulus activity is associated with better memory. We therefore hypothesized that voluntarily increasing activity in the PHC during word encoding will lead to improved memory for words. Our results, however, showed the opposite. Voluntarily increasing activity in the PHC led to decreased memory for words (Fig. 9).
This result is in stark contrast to the above mentioned studies which found that increased PHC activity correlated with better memory formation. However, similar to our results, a recent study also reported that decreased PHC activity led to better memory formation (Yoo et al., 2012). Using real-time fMRI, Yoo and colleagues monitored spontaneous fluctuations of PHC activity and triggered a memory probe depending on the activity level of the PHC. They found that memory probes that were triggered during decreased PHC activity levels were remembered significantly better than memory probes that were triggered during high PHC activity levels. They speculated that the lower levels of spontaneous PHC activity might reflect less processing, thus leaving more resources available for memory encoding. Likewise, our results could arise from a similar mechanism; voluntarily decreasing PHC activity could leave more resources available for memory encoding. Whereas Yoo et al. studied spontaneous fluctuations in PHC activity, the participants in our study voluntarily modulated PHC activity. It is thus possible that the cognitive strategy for up-regulating PHC activity competed for the same limited resources with memory encoding processes. For example, occupying the PHC with a spatial navigation strategy might withdraw resources important for verbal encoding. Such dual task interferences between self-regulation of brain activity and behavioral tasks have also been reported in other neurofeedback studies (Lutzenberger, Roberts, & Birbaumer, 1993; Rota et al., 2009). However, the participants in our study used comparable cognitive imagery in up- and down-regulation conditions (with respect to complexity and attentional demands). Hence, the interference that might underlie the present findings is related to neurofeedback-induced changes in specific neuronal populations of the PHC, rather than unspecific factors such as arousal or attention (Klingberg & Roland, 1997). Please note that we cannot completely exclude the possibility that the memory-related effects are partly due to activity changes in the SMA. However, given the functional specialization of our ROIs, such an explanation is unlikely.
5. Conclusion
Previous studies revealed that sustained brain activity that is not related to a particular stimulus or to task execution has an impact on cognitive function. Using a real-time fMRI neurofeedback approach, we extended this previous research by specifically training voluntary control over sustained brain activity to which we otherwise do not have conscious access to. Further, our novel experimental design allowed participants even to simultaneously learn bidirectional control over two functionally distinct brain regions. Control of these two regions caused characteristic behavioral effects that were related to the specific function of the brain region, i.e. reaction time changes were related to regulating activity in the SMA, and changes in memory performance were related to regulating activity in the PHC. Hence, the behavioral effects that we observed were due to changing regionally and functionally specific brain activity rather than to changes in general indices of cognitive efficiency such as attention or arousal. Because we did not test if the behavioral effects were also evident independent of actively self-regulating, we cannot conclude if there are lasting plastic changes that go beyond those related to temporarily increasing ongoing activity.
The sample size in our study was rather small and, as a consequence, the behavioral effects of particularly the reaction time task were somewhat statistically weak. Nonetheless, the faster responses when voluntarily increasing activity in the SMA might indicate the potential for cognitive enhancement through real-time fMRI-based brain training. Learning voluntary control of the SMA could also be used as a clinical treatment for Tourette's syndrome, where SMA activity is linked to motor tics (Bohlhalter et al., 2006; Hampson et al., 2011; Stern et al., 2000), or it could be used to promote recovery from stroke (Braun, Beurskens, Borm, Schack, & Wade, 2006; de Vries & Mulder, 2007; Sitaram et al., 2012). Also learning voluntary control over PHC activity might have important implications for a range of cognitive functions such as learning and memory (Asaka et al., 2005; Seager, Johnson, Chabot, Asaka, & Berry, 2002; Yoo et al., 2012), as well as neuropsychiatric conditions such as posttraumatic stress disorder (Geuze, Vermetten, Ruf, de Kloet, & Westenberg, 2008; Werner et al., 2008), schizophrenia (Bodnar et al., 2012; Diederen et al., 2010; Ragland et al., 2009), or Alzheimer's disease (Hyman, Van Hoesen, Damasio, & Barnes, 1984; Peters et al., 2009). However, in order to demonstrate the usefulness of this approach in clinical practice, follow-up patient studies with larger samples are needed.
Using neurofeedback to induce regionally specific changes in brain activity without drugs goes beyond conventional brain imaging studies that are only correlational. Similarly to other approaches that interfere with brain activity like transcranial magnetic stimulation, deep brain stimulation, neuropharmacological interventions, and brain lesions, it permits us to manipulate behavior causally.
Acknowledgements
This work was supported by the WIN-Program of the Heidelberg Academy of Sciences, the Swiss National Science Foundation, the European Union (Marie Curie Career Integration Grant FP7-PEOPLE-2011-CIG), the Wellcome Trust, the Deutsche Forschungsgemeinschaft (DFG grant BI 195/64-1), the Baden-Württemberg Stiftung, and the Stiftung Volkswagenwerk (VW). This work is part of the BRAINTRAIN European research network (Collaborative Project) supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (Grant agreement n° 602186). We thank Jürgen Dax, Michael Erb, Uta Benner, and Giuseppina Rota for help with this study.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Arai N., Lu M.K., Ugawa Y., Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: A paired-coil TMS study. Experimental Brain Research. 2012;220:79–87. doi: 10.1007/s00221-012-3117-5. [DOI] [PubMed] [Google Scholar]
- Arai N., Muller-Dahlhaus F., Murakami T., Bliem B., Lu M.K., Ugawa Y. State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. Journal of Neuroscience. 2011;31:15376–15383. doi: 10.1523/JNEUROSCI.2271-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A., Sterkin A., Grinvald A., Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Asaka Y., Mauldin K.N., Griffin A.L., Seager M.A., Shurell E., Berry S.D. Nonpharmacological amelioration of age-related learning deficits: The impact of hippocampal theta-triggered training. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13284–13288. doi: 10.1073/pnas.0506515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber T.X., Wilson S.C. The barber suggestibility scale and the creative imagination scale: Experimental and clinical applications. The American Journal of Clinical Hypnosis. 1978;21:84–108. doi: 10.1080/00029157.1978.10403966. [DOI] [PubMed] [Google Scholar]
- Birbaumer N., Ghanayim N., Hinterberger T., Iversen I., Kotchoubey B., Kubler A. A spelling device for the paralysed. Nature. 1999;398:297–298. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- Birbaumer N., Murguialday A.R., Cohen L. Brain–computer interface in paralysis. Current Opinion in Neurology. 2008;21:634–638. doi: 10.1097/WCO.0b013e328315ee2d. [DOI] [PubMed] [Google Scholar]
- Birbaumer N., Ruiz S., Sitaram R. Learned regulation of brain metabolism. Trends in Cognitive Sciences. 2013;17:295–302. doi: 10.1016/j.tics.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Bodnar M., Malla A.K., Joober R., Lord C., Smith E., Pruessner J. Neural markers of early remission in first-episode schizophrenia: A volumetric neuroimaging study of the parahippocampus. Psychiatry Research-Neuroimaging. 2012;201:40–47. doi: 10.1016/j.pscychresns.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S., Goldfine A., Matteson S., Garraux G., Hanakawa T., Kansaku K. Neural correlates of tic generation in Tourette syndrome: An event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Boly M., Balteau E., Schnakers C., Degueldre C., Moonen G., Luxen A. Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12187–12912. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S.M., Beurskens A.J., Borm P.J., Schack T., Wade D.T. The effects of mental practice in stroke rehabilitation: A systematic review. Archives of Physical Medicine and Rehabilitation. 2006;87:842–852. doi: 10.1016/j.apmr.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Bray S., Shimojo S., O’Doherty J.P. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. Journal of Neuroscience. 2007;27:7498–7507. doi: 10.1523/JNEUROSCI.2118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.B., Zhao Z., Desmond J.E., Glover G.H., Gabrieli J.D.E. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Broadbent D.E. Academic Press; London: 1971. Decision and stress. [Google Scholar]
- Caria A., Sitaram R., Veit R., Begliomini C., Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biological Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Caria A., Veit R., Sitaram R., Lotze M., Weiskopf N., Grodd W. Regulation of anterior insular cortex activity using real-time fMRI. NeuroImage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Chiew M., LaConte S.M., Graham S.J. Investigation of fMRI neurofeedback of differential primary motor cortex activity using kinesthetic motor imagery. NeuroImage. 2012;61:21–31. doi: 10.1016/j.neuroimage.2012.02.053. [DOI] [PubMed] [Google Scholar]
- de Vries S., Mulder T. Motor imagery and stroke rehabilitation: A critical discussion. Journal of Rehabilitation Medicine. 2007;39:5–13. doi: 10.2340/16501977-0020. [DOI] [PubMed] [Google Scholar]
- deCharms R.C. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends in Cognitive Sciences. 2007;11:473–481. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- deCharms R.C. Applications of real-time fMRI. Nature Reviews Neuroscience. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- deCharms R.C., Christoff K., Glover G.H., Pauly J.M., Whitfield S., Gabrieli J.D.E. Learned regulation of spatially localized brain activation using real-time fMRI. NeuroImage. 2004;21:436–443. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- deCharms R.C., Maeda F., Glover G.H., Ludlow D., Pauly J.M., Soneji D. Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R., Schwarzbauer C., Turner R. Optimisation of the 3DMDEFT sequence for anatomical brain imaging: technical implications at1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Diederen K.M.J., Neggers S.F.W., Daalman K., Blom J.D., Goekoop R., Kahn R.S. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. American Journal of Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- Dunn T.G., Gillig S.E., Ponsor S.E., Weil N., Utz S.W. The learning-process in biofeedback—Is it feedforward or feedback? Biofeedback and Self-Regulation. 1986;11:143–156. doi: 10.1007/BF00999982. [DOI] [PubMed] [Google Scholar]
- Fernandez G., Brewer J.B., Zhao Z., Glover G.H., Gabrieli J.D.E. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: A functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fetz E.E. Operant conditioning of cortical unit activity. Science. 1969;163:955-&. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- Fetz E.E. Volitional control of neural activity: Implications for brain–computer interfaces. Journal of Physiology-London. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Freeman G.L. The facilitative and inhibitory effects of muscular tension upon performance. American Journal of Psychology. 1933;45:17–52. [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fuchs T., Birbaumer N., Lutzenberger W., Gruzelier J.H., Kaiser J. Neurofeedback treatment for attention–deficit/hyperactivity disorder in children: A comparison with methylphenidate. Applied Psychophysiology and Biofeedback. 2003;28:1–12. doi: 10.1023/a:1022353731579. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Brewer J.B., Desmond J.E., Glover G.H. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Geuze E., Vermetten E., Ruf M., de Kloet C.S., Westenberg H.G.M. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gevensleben H., Rothenberger A., Moll G.H., Heinrich H. Neurofeedback in children with ADHD: Validation and challenges. Expert Review of Neurotherapeutics. 2012;12:447–460. doi: 10.1586/ern.12.22. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Eickhoff S.B., Nowak D.A., Dafotakis M., Fink G.R. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage. 2008;41:1382–1394. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Guillot A., Collet C., Nguyen V.A., Malouin F., Richards C., Doyon J. Brain activity during visual versus kinesthetic imagery: An fMRI study. Human Brain Mapping. 2009;30:2157–2172. doi: 10.1002/hbm.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S., Birbaumer N., Veit R. Real-time fMRI feedback training may improve chronic tinnitus. European Radiology. 2010;20:696–703. doi: 10.1007/s00330-009-1595-z. [DOI] [PubMed] [Google Scholar]
- Haller S., Kopel R., Jhooti P., Haas T., Scharnowski F., Lovblad K.O. Dynamic reconfiguration of human brain functional networks through neurofeedback. NeuroImage. 2013;81:243–252. doi: 10.1016/j.neuroimage.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Hamada M., Hanajima R., Terao Y., Okabe S., Nakatani-Enomoto S., Furubayashi T. Primary motor cortical metaplasticity induced by priming over the supplementary motor area. Journal of Physiology-London. 2009;587:4845–4862. doi: 10.1113/jphysiol.2009.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Glover G.H., Hsu J.-J., Johnson R.F., Gotlib I.H. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Scheinost D., Qiu M., Bhawnani J., Lacadie C.M., Leckman J.F. Biofeedback of real-time functional magnetic resonance imaging data from the supplementary motor area reduces functional connectivity to subcortical regions. Brain Connectivity. 2011;1:91–98. doi: 10.1089/brain.2011.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G., Kell C.A., Eger E., Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10984–10989. doi: 10.1073/pnas.0712043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G., Kell C.A., Kleinschmidt A. Ongoing activity fluctuations in hMT plus bias the perception of coherent visual motion. Journal of Neuroscience. 2008;28:14481–14485. doi: 10.1523/JNEUROSCI.4398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B., Van Hoesen G., Damasio A., Barnes C. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- James W. Holt; New York, NY: 1890. The Principles of psychology. [Google Scholar]
- Johansen-Berg H., Behrens T.E.J., Robson M.D., Drobnjak I., Rushworth M.F.S., Brady J.M. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Hartwell K., LeMatty T., Borckardt J., Morgan P.S., Govindarajan K. Intermittent real-time fMRI feedback is superior to continuous presentation for a motor imagery task: A pilot study. Journal of Neuroimaging. 2012;22:58–66. doi: 10.1111/j.1552-6569.2010.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Linden D.E.J., Healy D., Goebel R., Habes I., Boehm S.G. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cognitive Affective & Behavioral Neuroscience. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- Johnston S.J., Boehm S.G., Healy D., Goebel R., Linden D.E.J. Neurofeedback: A promising tool for the self-regulation of emotion networks. NeuroImage. 2010;49:1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Kennerley S.W., Sakai K., Rushworth M.F. Organization of action sequences and the role of the pre-SMA. Journal of Neurophysiology. 2004;91:978–993. doi: 10.1152/jn.00651.2003. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Roland P.E. Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Cognitive Brain Research. 1997;6:1–8. doi: 10.1016/s0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Koeneke S., Lutz K., Wustenberg T., Jancke L. Bimanual versus unimanual coordination: What makes the difference? NeuroImage. 2004;22:1336–1350. doi: 10.1016/j.neuroimage.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Kosslyn S.M., Ganis G., Thompson W.L. Neural foundations of imagery. Nature Reviews Neuroscience. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B., Kubler A., Strehl U., Flor H., Birbaumer N. Can humans perceive their brain states? Consciousness and Cognition. 2002;11:98–113. doi: 10.1006/ccog.2001.0535. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B., Strehl U., Uhlmann C., Holzapfel S., Konig M., Froscher W. Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia. 2001;42:406–416. doi: 10.1046/j.1528-1157.2001.22200.x. [DOI] [PubMed] [Google Scholar]
- Koush Y., Zvyagintsev M., Dyck M., Mathiak K.A., Mathiak K. Signal quality and Bayesian signal processing in neurofeedback based on real-time fMRI. NeuroImage. 2012;59:478–489. doi: 10.1016/j.neuroimage.2011.07.076. [DOI] [PubMed] [Google Scholar]
- Kübler A., Kotchoubey B., Kaiser J., Wolpaw J.R., Birbaumer N. Brain–computer communication: Unlocking the locked in. Psychological Bulletin. 2001;127:358–375. doi: 10.1037/0033-2909.127.3.358. [DOI] [PubMed] [Google Scholar]
- Lacroix J.M., Roberts L.E. Comparison of mechanisms and some properties of instructed sudomotor and cardiac control. Biofeedback and Self-Regulation. 1978;3:105–132. doi: 10.1007/BF00998897. [DOI] [PubMed] [Google Scholar]
- Linden D.E.J., Habes I., Johnston S.J., Linden S., Tatineni R., Subramanian L. Real-time self-regulation of emotion networks in patients with depression. PLoS ONE. 2012;7(6):7. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G., Matelli M., Camarda R., Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (Pre-SMA) in the macaque monkey. Journal of Comparative Neurology. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W., Roberts L.E., Birbaumer N. Memory performance and area-specific self-regulation of slow cortical potentials—Dual-task interference. International Journal of Psychophysiology. 1993;15:217–226. doi: 10.1016/0167-8760(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Mathiak K.A., Koush Y., Dyck M., Gaber T.J., Alawi E., Zepf F.D. Social reinforcement can regulate localized brain activity. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:S132–S136. doi: 10.1007/s00406-010-0135-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto R., Nair D.R., LaPresto E., Bingaman W., Shibasaki H., Luders H.O. Functional connectivity in human cortical motor system: A cortico-cortical evoked potential study. Brain. 2007;130:181–197. doi: 10.1093/brain/awl257. [DOI] [PubMed] [Google Scholar]
- Matsunaga K., Maruyama A., Fujiwara T., Nakanishi R., Tsuji S., Rothwell J.C. Increased corticospinal excitability after 5 Hz rTMS over the human supplementary motor area. Journal of Physiology-London. 2005;562:295–306. doi: 10.1113/jphysiol.2004.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T.S., Polanczyk G., Caye A., Banaschewski T., Brandeis D., Rohde L.A. Evidence-based information on the clinical use of neurofeedback for ADHD. Neurotherapeutics. 2012;9:588–598. doi: 10.1007/s13311-012-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Neumann N., Kubler A., Kaiser J., Hinterberger T., Birbaumer N. Conscious perception of brain states: Mental strategies for brain–computer communication. Neuropsychologia. 2003;41:1028–1036. doi: 10.1016/s0028-3932(02)00298-1. [DOI] [PubMed] [Google Scholar]
- O’Craven K.M., Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience. 2000;12:1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Olds J. Operant conditioning of single unit responses. Expcerpta Med Int Cogn Series. 1965;87:372–380. [Google Scholar]
- Otten L.J., Quayle A.H., Akram S., Ditewig T.A., Rugg M.D. Brain activity before an event predicts later recollection. Nature Neuroscience. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- Peters F., Collette F., Degueldre C., Sterpenich V., Majerus S., Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: An fMRI study. Brain. 2009;132:1833–1846. doi: 10.1093/brain/awp075. [DOI] [PubMed] [Google Scholar]
- Ragland J.D., Laird A.R., Ranganath C., Blumenfeld R.S., Gonzales S.M., Glahn D.C. Prefrontal activation deficits during episodic memory in Schizophrenia. American Journal of Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ress D., Backus B.T., Heeger D.J. Activity in primary visual cortex predicts performance in a visual detection task. Nature Neuroscience. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Roberts L.E., Birbaumer N., Rockstroh B., Lutzenberger W., Elbert T. Self-report during feedback-regulation of slow cortical potentials. Psychophysiology. 1989;26:392–403. doi: 10.1111/j.1469-8986.1989.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Roberts L.E., Williams R.J., Marlin R.G., Farrell T., Imiolo D. Awareness of the response after feedback training for changes in heart-rate and sudomotor laterality. Journal of Experimental Psychology-General. 1984;113:225–255. doi: 10.1037//0096-3445.113.2.225. [DOI] [PubMed] [Google Scholar]
- Robineau F., Rieger S.W., Mermoud C., Pichon S., Koush Y., Van De Ville D. Self-regulation of inter-hemispheric visual cortex balance through real-time fMRI neurofeedback training. NeuroImage. 2014;100:1–14. doi: 10.1016/j.neuroimage.2014.05.072. [DOI] [PubMed] [Google Scholar]
- Rota G., Handjaras G., Sitaram R., Birbaumer N., Dogil G. Reorganization of functional and effective connectivity during real-time fMRI-BCI modulation of prosody processing. Brain and Language. 2011;117:123–132. doi: 10.1016/j.bandl.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Rota G., Sitaram R., Veit R., Erb M., Weiskopf N., Dogil G. Self-regulation of regional cortical activity using real-time fMRI: The right inferior frontal gyrus and linguistic processing. Human Brain Mapping. 2009;30:1605–1614. doi: 10.1002/hbm.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnowski F., Hutton C., Josephs O., Weiskopf N., Rees G. Improving visual perception through neurofeedback. Journal of Neuroscience. 2012;32:17830–17841. doi: 10.1523/JNEUROSCI.6334-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober R., Lacroix J.M. Effects of task instructions and contingency on the development of phasic heart-rate control and its correlates. Canadian Journal of Psychology-Revue Canadienne De Psychologie. 1986;40:54–64. doi: 10.1037/h0080085. [DOI] [PubMed] [Google Scholar]
- Seager M.A., Johnson L.D., Chabot E.S., Asaka Y., Berry S.D. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Watanabe T., Sasaki Y., Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334:1413–1415. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y., Hamada M., Terao Y., Ohminami S., Tsutsumi R., Ugawa Y. ET-AL>. Increased primary motor cortical excitability by a single-pulse transcranial magnetic stimulation over the supplementary motor area. Experimental Brain Research. 2012;219:339–349. doi: 10.1007/s00221-012-3095-7. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M., Kropp P., Gerber W.D. Neurofeedback—The significance of reinforcement and the search for an appropriate strategy for the success of self-regulation. Applied Psychophysiology and Biofeedback. 2000;25:167–175. doi: 10.1023/a:1009502808906. [DOI] [PubMed] [Google Scholar]
- Sitaram R., Veit R., Stevens B., Caria A., Gerloff C., Birbaumer N. Acquired control of ventral premotor cortex activity by feedback training: An exploratory real-time FMRI and TMS study. Neurorehabilitation and Neural Repair. 2012;26:256–265. doi: 10.1177/1545968311418345. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Thompson W.L., Kosslyn S.M. Visual mental imagery induces retniotopically organized activation of early visual areas. Cerebral Cortex. 2005;15:1570–1583. doi: 10.1093/cercor/bhi035. [DOI] [PubMed] [Google Scholar]
- Sterman M.B., Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback. 2006;31:21–35. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Stern C.E., Corkin S., Gonzalez R.G., Guimaraes A.R., Baker J.R., Jennings P.J. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E., Silbersweig D.A., Chee K.Y., Holmes A., Robertson M.M., Trimble M. A functional neuroanatomy of ties in Tourette syndrome. Archives of General Psychiatry. 2000;57:741–748. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- Stokes M., Thompson R., Cusack R., Duncan J. Top-down activation of shape-specific population codes in visual cortex during mental imagery. Journal of Neuroscience. 2009;29:1565–1572. doi: 10.1523/JNEUROSCI.4657-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf H., Fay E. Hogrefe; Göttingen: 1983. Schlauchfiguren: Ein Test zur Beurteilung des räumlichen Vorstellungsvermögens (Manual) [Google Scholar]
- Subramanian L., Hindle J.V., Johnston S., Roberts M.V., Husain M., Goebel R. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson's disease. The Journal of Neuroscience. 2011;31:16309–16317. doi: 10.1523/JNEUROSCI.3498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer J., Haller S., Scharnowski F., Weiskopf N., Birbaumer N., Blefari M.L. Real-time fMRI neurofeedback: Progress and challenges. NeuroImage. 2013;76C:386–399. doi: 10.1016/j.neuroimage.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer J., Sitaram R., Blefari M.L., Kollias S., Birbaumer N., Stephan K.E. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. NeuroImage. 2013;(83):817–825. doi: 10.1016/j.neuroimage.2013.05.115. [DOI] [PubMed] [Google Scholar]
- Tan G., Thornby J., Hammond D.C., Strehl U., Canady B., Arnemann K. Meta-analysis of EEG biofeedback in treating epilepsy. Clinical EEG and Neuroscience. 2009;40:173–179. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- Tanji J. Sequential organization of multiple movements: Involvement of cortical motor areas. Annual Review of Neuroscience. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- Tanji J., Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Tokuno H., Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: An electrophysiological study in the macaque monkey. Cerebral Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Turk-Browne N.B., Yi D.J., Chun M.M. Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Utz S.W. The effect of instructions derived from 2 models of learning on cognitive strategies and EMG biofeedback. Biofeedback and Self-Regulation. 1987;12:165–166. [Google Scholar]
- Utz S.W. The effect of instructions on cognitive strategies and performance in biofeedback. Journal of Behavioral Medicine. 1994;17:291–308. doi: 10.1007/BF01857954. [DOI] [PubMed] [Google Scholar]
- Veit R., Singh V., Sitaram R., Caria A., Rauss K., Birbaumer N. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Social Cognitive and Affective Neuroscience. 2012;7:623–634. doi: 10.1093/scan/nsr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Schacter D.L., Rotte M., Koutstaal W., Maril A., Dale A.M. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Scharnowski F., Veit R., Goebel R., Birbaumer N., Mathiak K. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI) Journal of Physiology-Paris. 2004;98:357–373. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Sitaram R., Josephs O., Veit R., Scharnowski F., Goebel R. Real-time functional magnetic resonance imaging: Methods and applications. Magnetic Resonance Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Veit R., Erb M., Mathiak K., Grodd W., Goebel R. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): Methodology and exemplary data. NeuroImage. 2003;19:577–586. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Werner N.S., Meindl T., Engel R.R., Rosner R., Riedel M., Reiser M. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;43:309–318. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Wiestler T., Diedrichsen J. Skill learning strengthens cortical representations of motor sequences. Elife. 2013;2:e00801. doi: 10.7554/eLife.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wundt W. Ueber die Messung psychischer Zustaende. Philosophische Studien. 1882;1:251–260. [Google Scholar]
- Yanaka H.T., Saito D.N., Uchiyama Y., Sadato N. Neural substrates of phasic alertness: A functional magnetic resonance imaging study. Neurosci Res. 2010;68:51–58. doi: 10.1016/j.neures.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Yoo J.J., Hinds O., Ofen N., Thompson T.W., Whitfield-Gabrieli S., Triantafyllou C. When the brain is prepared to learn: Enhancing human learning using real-time fMRI. NeuroImage. 2012;59:846–852. doi: 10.1016/j.neuroimage.2011.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.-S., Lee J.-H., O’Leary H., Lee V., Choo S.-E., Jolesz F.A. Functional magnetic resonance imaging-mediated learning of increased activity in auditory areas. NeuroReport. 2007;18:1915–1920. doi: 10.1097/WNR.0b013e3282f202ac. [DOI] [PubMed] [Google Scholar]
- Yoo S.-S., Lee J.-H., O’Leary H., Panych L.P., Jolesz F.A. Neurofeedback fMRI-mediated learning and consolidation of regional brain activation during motor imagery. International Journal of Imaging Systems and Technology. 2008;18:69–78. doi: 10.1002/ima.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerssen v.B. Beltz Test; Weinheim: 1976. Die Befindlichkeitsskala (Bf-S)—Manual. [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P. Self-regulation of amygdala activation using real-time fMRI neurofeedback. PLoS ONE. 2011;6(9):6. doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.