Abstract
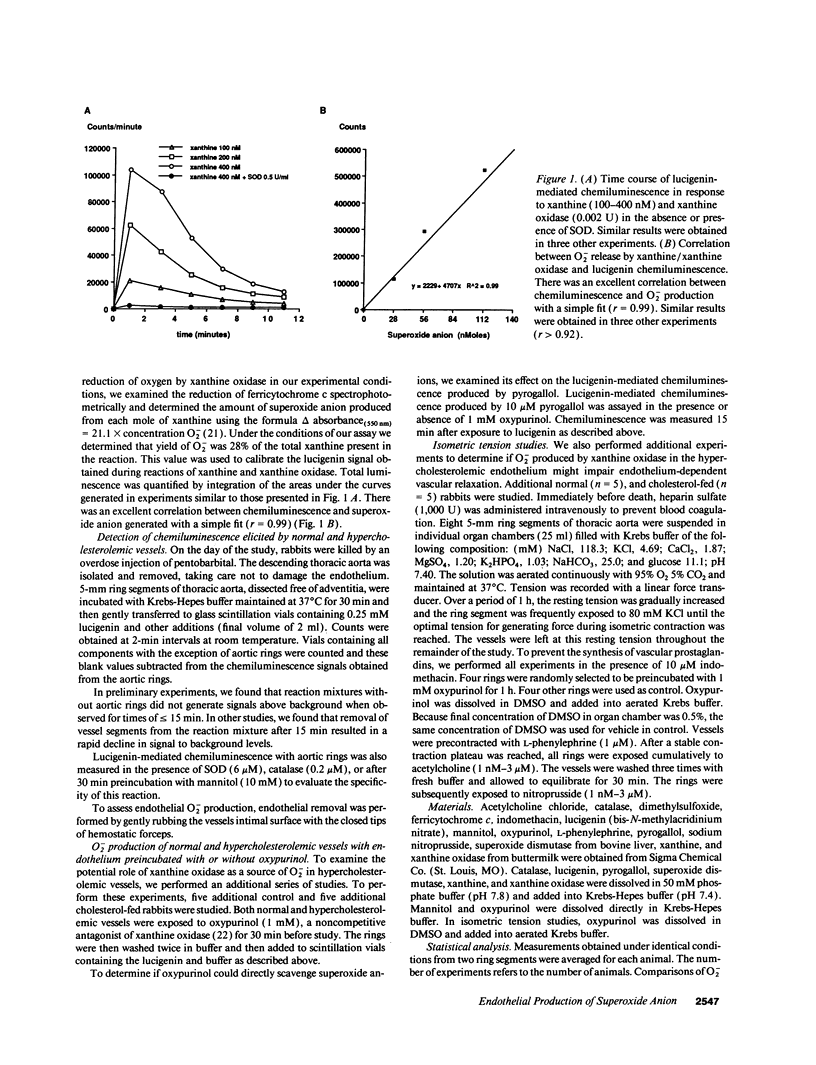
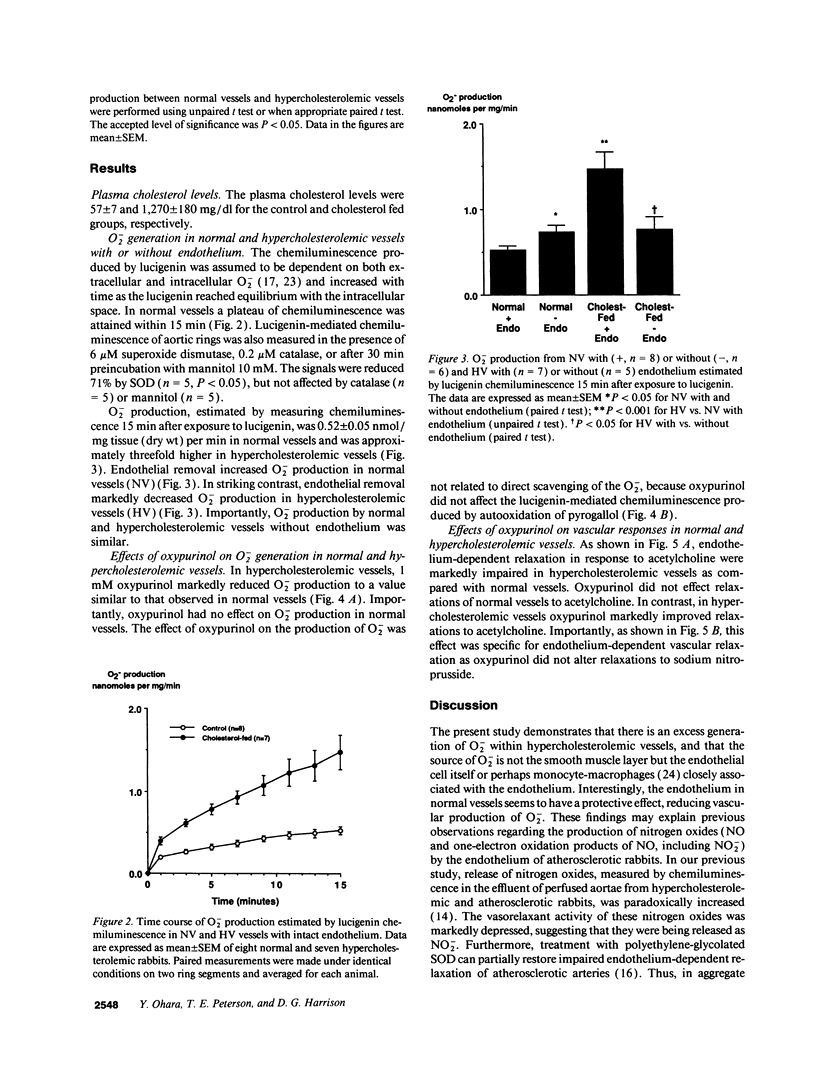
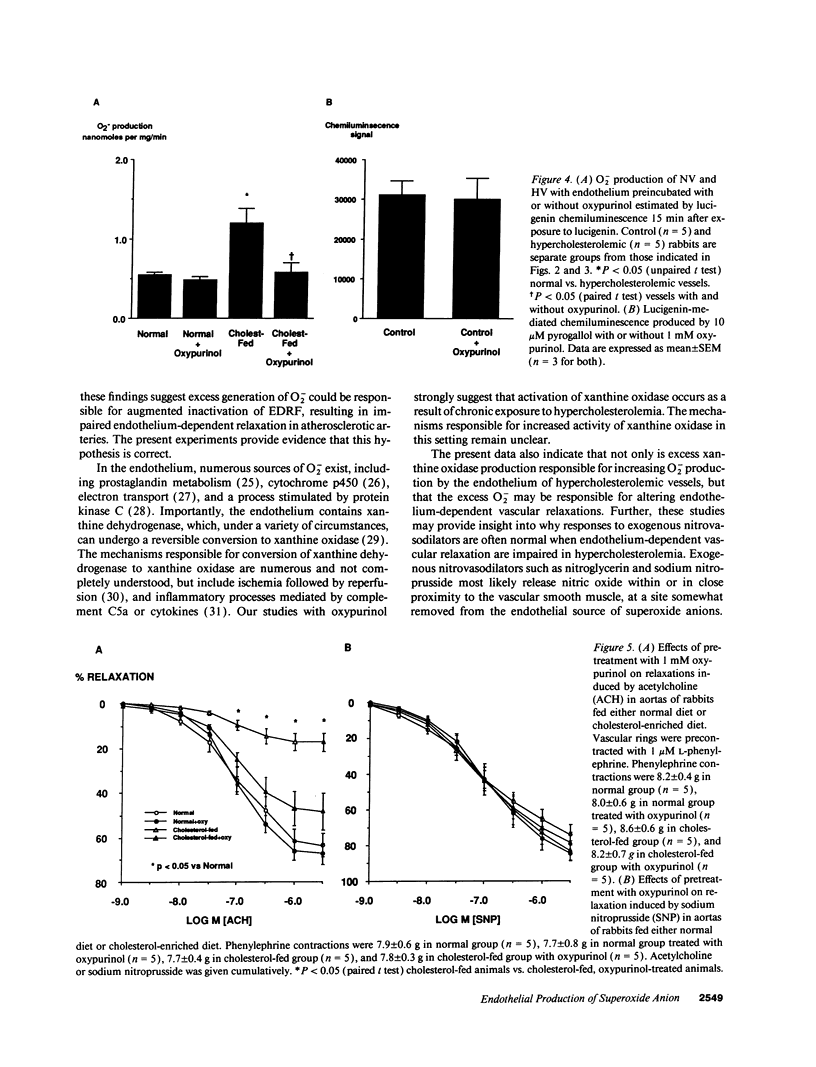
Indirect evidence suggests accelerated degradation of endothelium-derived nitric oxide (ENDO) by superoxide anion (O2-) in hypercholesterolemic vessels (HV). To directly measure O2- production by normal vessels (NV) and HV, we used an assay for O2- based on the chemiluminescence (CL) of lucigenin (L). HV (1 mo cholesterol-fed rabbits) produced threefold more O2- than NV (1.47 +/- 0.20 nM/mg tissue/min, n = 7 vs. 0.52 +/- 0.05 nmol/mg tissue/min, n = 8, P < 0.001). Endothelial removal increased O2- production in NV (0.73 +/- 0.08, n = 6, P < 0.05), while decreasing it in HV (0.76 +/- 0.15, n = 5, P < 0.05). There was no difference between denuded HV and denuded NV. Oxypurinol, a noncompetitive inhibitor of xanthine oxidase, normalized O2- production in HV, but had no effect in NV. In separate isometric tension studies treatment with oxypurinol improved acetylcholine induced relaxations in HV, while having no effect on responses in normal vessels. Oxypurinol did not alter relaxations to nitroprusside. Thus, the endothelium is a source of O2- in hypercholesterolemia probably via xanthine oxidase activation. Increased endothelial O2- production in HV may inactivate endothelium-derived nitric oxide and provide a source for other oxygen radicals, contributing to the early atherosclerotic process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry P. D., Omar H. A., Farrell K. A., Stuart J. S., Wolin M. S. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol. 1990 Oct;259(4 Pt 2):H1056–H1062. doi: 10.1152/ajpheart.1990.259.4.H1056. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cera E., Doyle M. L., Gill S. J. Alkaline Bohr effect of human hemoglobin Ao. J Mol Biol. 1988 Apr 5;200(3):593–599. doi: 10.1016/0022-2836(88)90545-1. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Kovensky A., Hitchings G. H. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966 Jul;15(7):863–880. doi: 10.1016/0006-2952(66)90163-8. [DOI] [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987 Mar 12;97(2):209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- Heim K. F., Thomas G., Ramwell P. W. Superoxide production in the isolated rabbit aorta and the effect of alloxan, indomethacin and nitrovasodilators. J Pharmacol Exp Ther. 1991 Feb;256(2):537–541. [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988 Jan;244(1):181–189. [PubMed] [Google Scholar]
- Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can J Physiol Pharmacol. 1985 Sep;63(9):1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Ellis E. F., Jenkins L. W., Povlishock J. T., Rowe G. T., Hess M. L. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ Res. 1985 Jul;57(1):142–151. doi: 10.1161/01.res.57.1.142. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982 Nov 5;218(4572):576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- Kubo S. H., Rector T. S., Bank A. J., Williams R. E., Heifetz S. M. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991 Oct;84(4):1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- Lamping K. G., Dole W. P. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circ Res. 1987 Dec;61(6):904–913. doi: 10.1161/01.res.61.6.904. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- Melinn M., McLaughlin H. Nitroblue tetrazolium reduction in lymphocytes. J Leukoc Biol. 1987 Apr;41(4):325–329. doi: 10.1002/jlb.41.4.325. [DOI] [PubMed] [Google Scholar]
- Minor R. L., Jr, Myers P. R., Guerra R., Jr, Bates J. N., Harrison D. G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990 Dec;86(6):2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Harrison D. G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991 Feb;260(2 Pt 1):C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Omar H. A., Cherry P. D., Mortelliti M. P., Burke-Wolin T., Wolin M. S. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res. 1991 Sep;69(3):601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Panza J. A., Quyyumi A. A., Brush J. E., Jr, Epstein S. E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990 Jul 5;323(1):22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Sligar S. G., Lipscomb J. D., Debrunner P. G., Gunsalus I. C. Superoxide anion production by the autoxidation of cytochrome P450cam. Biochem Biophys Res Commun. 1974 Nov 6;61(1):290–296. doi: 10.1016/0006-291x(74)90565-8. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988 Mar 4;959(1):20–30. doi: 10.1016/0005-2760(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Superoxide generation by human monocytes and macrophages. Am J Hematol. 1978;4(1):1–8. doi: 10.1002/ajh.2830040102. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


