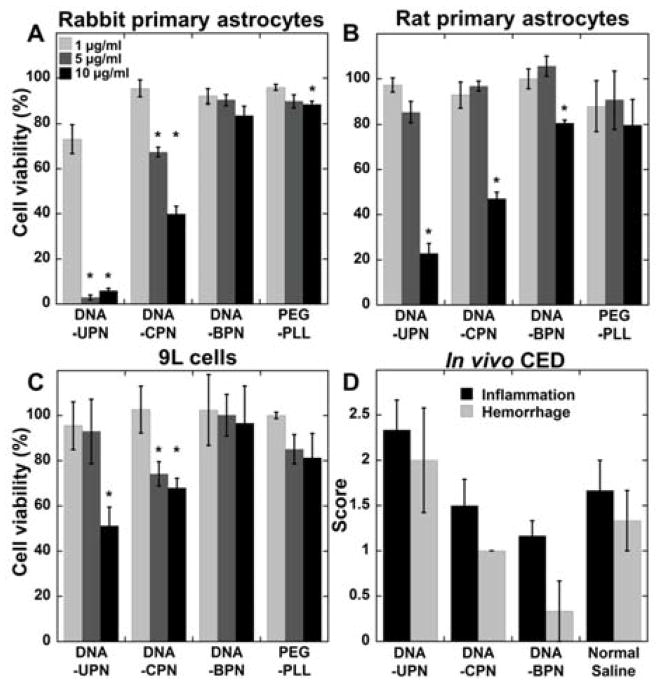
Figure 2. Safety profile of gene vectors.
(A) Rabbit primary astrocytes, (B) rat primary astrocytes and (C) 9L rat gliosarcoma cells were treated with varying concentrations of cationic polymer-based gene vectors. Cell viability was measured after 24 h of treatment and compared to untreated controls. Data represented as mean ± SEM. *Denotes statistically significant difference from 100% viability (p < 0.05). (D) Histopathological analysis of gene vector safety profile following CED administration. Normal saline was used as a control. Inflammation and hemorrhage were scored by a board certified neuropathologist using a custom scale (0: no inflammation/hemorrhage, 1: mild, 2: moderate, 3: severe).