Fig 1. Secretion of the yopD mutants.
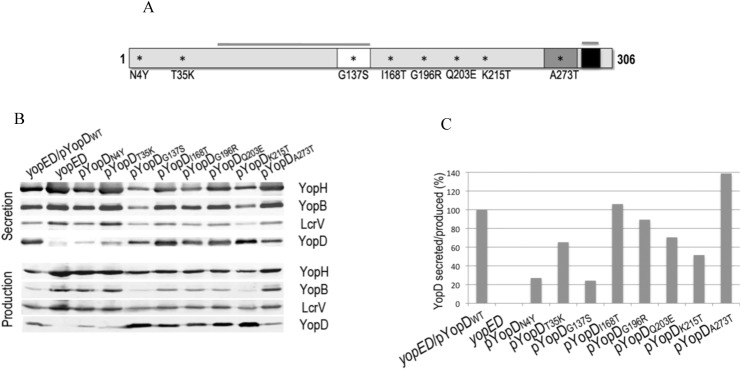
A. Schematic representation of YopD protein indicating a predicted transmembrane domain (white), a putative coiled-coil region (dark grey) and an amphipathic α-helix domain (black). Overlined are the N-terminal and C-terminal chaperon-binding regions (53–149 and 278–292). Amino acid substitutions that interfere with pore formation, translocation or both are indicated by asterisks. B. Immunoblot showing Yop secretion (bacterial supernatant) and production (bacterial lysate) for each of the YopD mutants expressed in a yopED background and grown at 37°C at low calcium conditions. Monoclonal antibodies for the detection of YopD and YopB and polyclonal antibodies for YopH and LcrV are described in Material and Methods. Anti-rabbit and anti-mouse IR680 or IR800 were used as secondary antibodies. Infrared signal was detected using the Odyssey imaging system (LI-COR Biosience). Quantification of the YopD signal intensities was performed using Odyssey imaging system software. The ratio between the amount of YopD secreted and YopD produced was calculated for each mutant and normalized to wild type YopD.
