BMI was not significantly associated with clinical outcomes among patients with DLBCL, HD or FL, in three prospective phase III clinical trials.
Keywords: body mass index, survival outcomes, diffuse large B-cell lymphoma, follicular lymphoma, Hodgkin's lymphoma
Abstract
Background
The role of body mass index (BMI) in survival outcomes is controversial among lymphoma patients. We evaluated the association between BMI at study entry and failure-free survival (FFS) and overall survival (OS) in three phase III clinical trials, among patients with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and Hodgkin's lymphoma (HL).
Patients and methods
A total of 537, 730 and 282 patients with DLBCL, HL and FL were included in the analysis. Baseline patient and clinical characteristics, treatment received and clinical outcomes were compared across BMI categories.
Results
Among patients with DLBCL, HL and FL, the median age was 70, 33 and 56; 29%, 29% and 37% were obese and 38%, 27% and 37% were overweight, respectively. Age was significantly different among BMI groups in all three studies. Higher BMI groups tended to have more favorable prognosis factors at study entry among DLBCL and HL patients. BMI was not associated with clinical outcome with P-values of 0.89, 0.30 and 0.40 for FFS, and 0.64, 0.67 and 0.09 for OS, for patients with DLBCL, HL and FL, respectively. The association remains non-significant after adjusting for other clinical factors in the Cox model. A subset analysis of males with DLBCL treated on R-CHOP revealed no differences in FFS (P = 0.48) or OS (P = 0.58).
Conclusion
BMI was not significantly associated with clinical outcomes among patients with DLBCL, HD or FL, in three prospective phase III clinical trials. The findings contradict some previous reports of similar investigations. Further work is required to understand the observed discrepancies.
introduction
Body mass index (BMI), calculated as the weight (kg) divided by the square of height (m), is commonly used as a reliable indicator of body fat content and is used to screen for weight categories that may lead to health problems. Obesity, defined as BMI ≥ 30 kg/m2, is a major public health problem in the United States; about two-thirds of the adult population is overweight or obese in 1999–2008 [1]. During the same period, cancer incidence rates have risen for most cancer types [2]. Population-based observational studies have linked increasing BMI to increasing risk of developing a number of common and some less common cancers, including both solid and hematologic malignancies [3, 4]. However, the impact of BMI on survival after cancer diagnosis is controversial.
In solid tumors, increased BMI was reported to associate with decreased survival probability and increased risk for recurrence among patients with breast cancer [5] and colon cancer [6] and to associate with improved survival among patients with renal cell carcinoma [7] and lung cancers [8]. In hematological malignancies, studies show that increased BMI does not confer a negative impact on the survival of obese multiple myeloma patients [9]. Mixed results have been reported investigating the effect of BMI on lymphoma prognosis. High BMI was associated with a worse prognosis and a greater risk for disease recurrence for non-Hodgkin’s lymphoma (NHL) patients receiving high-dose chemotherapy and autograft [10]. Landgren et al. [11] showed that 5-year cause-specific mortality rate was significantly lower among obese and overweight patients with Hodgkin's lymphoma (HL). Two recent studies suggest that increased BMI is associated with significantly improved survival, one among patients with intermediate-grade B-cell NHL receiving chemotherapy [12] and one among United States Veterans with diffuse large B-Cell Lymphoma (DLBCL) [13]. Both of the studies were retrospectively done with medical record review and with mixed histology and/or mixed first-line therapies.
In this study, we evaluated the association between BMI at study entry and failure-free survival (FFS) and overall survival (OS) in three prospectively designed phase III Cooperative Group trials, among patients with DLBCL (E4494), follicular lymphoma (FL) (E1496) and HL (E2496).
patients and methods
patients and treatment
The study included patients enrolled on three NCI-sponsored, phase III clinical trials coordinated by the Eastern Cooperative Oncology Group (ECOG). In E4494, 546 eligible untreated DLBCL patients, 60 years or older, enrolled between December 1997 to July 2001, were randomized to receive cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or rituximab plus CHOP (RCHOP), all responding patients (n = 352) went through a second randomization to maintenance rituximab (MR) or observation (OBS) [14]. In E1496, 387 eligible patients with advanced indolent lymphoma (282 follicular histology), enrolled from March 1998 to May 2003, received cyclophosphamide, vincristine and prednisone (CVP), 311 (228 with follicular histology) with responding or stable disease were randomized to observation (OBS) or rituximab [15]. In E2496, 794 eligible patients with advanced stage or locally extensive HL, enrolled between April 1999 and June 2006, were randomized to ABVD or Stanford V. Other details regarding eligibility, treatment received and results have been previously reported [16]. Chemotherapy was administered based on body surface area (BSA) from actual body weight, and vincristine was capped at 2 mg in E4494 and E1496. Thirty-seven (5%) cases in E2496, three cases in E1496 with missing baseline weight or height information were removed from the analysis (supplementary Figure S1, available at Annals of Oncology online). Only patients with follicular histology from E1496 were included for this study.
measurements
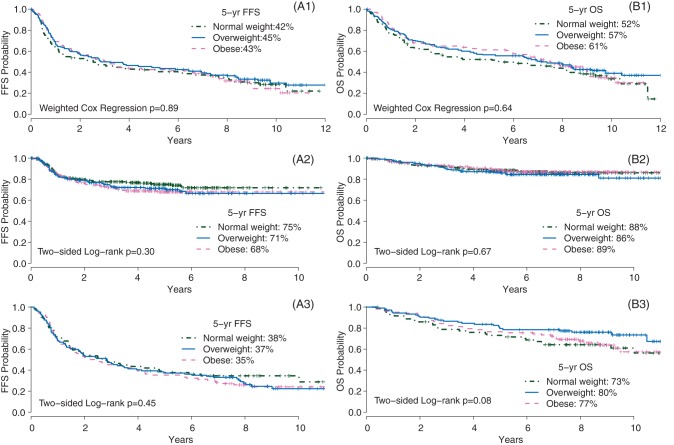
BMI was calculated as weight (kilograms) divided by the square of height (meters), using data at study entry. BMI was categorized according to the WHO classification: underweight (BMI < 18.5 kg/m2), normal weight (BMI, 18.5 to <25 kg/m2), overweight (BMI, 25 to <30 kg/m2) and obese (BMI, ≥ 30 kg/m2). Patients in the underweight group, consisting of 9 (2%) DLBCL patients, 18 (2%) HL patients and 0 FL patients, were excluded from further analysis due to low prevalence (Figure 1).
Figure 1.
Failure-free survival (FFS, A) and overall survival (OS, B) by BMI groups, for DLBCL patients enrolled in the E4494 trial with (A1, B1) (by weighted Cox regression model), for the HL patients in E2496 trial with (A2, B2) and the FL patients in E1496 trial with (A3, B3).
Baseline demographic/clinical factors and treatment data were obtained from the original study. The primary outcome measures are FFS or progression-free survival (PFS) and OS. FFS/PFS, though named differently in different studies, was both defined as the time from study entry to relapse, progression, or death, whichever occurred first. Herein, FFS is used in the text. OS was measured from study entry to death of any cause.
statistical analysis
Baseline patient characteristics were checked among three BMI groups, with the χ2 test for proportions and one-way analysis of variance for means, respectively. The Kaplan–Meier method and the Cox proportional hazards regression model were used to estimate survival curves, failure rate and hazard ratios with 95% confidence intervals (CIs). Log-rank tests, stratified on initial trial stratification factors and treatment arms, were used to compare among BMI groups. A multivariable Cox regression was used to further evaluate the association between BMI and FFS/OS, adjusting for known baseline prognostic factors. Possible two-way interaction between BMI and other factors was checked. BMI was studied both as continuous variable and categorical BMI groups. Cases with extreme BMI were removed from the analysis when BMI was checked as continuous variable. Based on the rule of 1.5 inter-quartile ranges, extreme BMI was defined as 50 or higher in all three data sets, which includes 1 case in E4494, 14 cases in E2496 and 3 cases in E1496. Two-sided P-values of 0.05 or less were considered statistically significant, and of 0.1 or less suggestive of a trend.
To control for treatment during the rituximab era, the association was also checked limiting to patients treated with rituximab: FL patients treated on rituximab maintenance from E1496 and DLBCL patients treated on RCHOP from E4494. To remove potential effect of disease-related weight loss in BMI determination, a sensitivity analysis was carried out excluding patients with significant weight loss at baseline.
In E4494, a significant interaction was identified between maintenance therapy and induction therapy where MR improved the outcome after CHOP but not after RCHOP. Therefore, an unbiased estimate by weighted Cox regression, as previously reported [14], was used to compare FFS/OS among BMI groups, by removing the effect of maintenance Rituximab. As a significant interaction between sex and treatment arm was identified among DLBCL patient [17], a separate analysis was carried out within each sex stratified on treatment arms. We analyzed the male subset under RCHOP treatment from E4494, in order to match the study cohort used in a recent report [13].
results
diffuse large B-cell lymphoma
Among a total of 537 DLBCL patients, 38% were overweight and 29% were obese. Obese group was significantly younger (P = 0.0002) than the normal and overweight groups. Overweight and obese groups had a trend toward better performance status compared with the normal weight group (P = 0.10). However, this trend disappeared if excluding patients with significant disease-related weight loss at baseline (P = 0.24). Other baseline factors and treatment received are similar among three BMI groups (supplementary Table S1, available at Annals of Oncology online). With a median follow-up of 9.4 years, weighted Cox regression analysis (Figure 1, Table 1) indicated that BMI groups are not significantly associated with FFS (P = 0.89) or with OS (P = 0.64). The hazard ratio for mortality was 0.85 (95% CI, 0.61–1.19) for overweight, and 0.89 (95% CI, 0.64–1.26) for obese, when compared with normal weight (Table 1). Using continuous BMI in the univariate Cox model also results in non-significant association with OS (P = 0.59, Table 1). When adjusting for known prognostic factors in the multivariable Cox model, interaction between sex and induction therapy (RCHOP versus CHOP) was found marginally significant (P = 0.09) and was retained in the model. BMI remains non-significant in associating with OS outcome, both as continuous (P = 0.96) and as categorical (P = 0.52, overweight versus normal weight; P = 0.96, obese versus normal weight, respectively).
Table 1.
Hazard ratio and 95% CIs for mortality associated with BMI as continuous variable and categorical variables, according to the Cox model
| DLBCL (E4494)a |
HL (E2496) |
FL (E1496) |
|||||
|---|---|---|---|---|---|---|---|
| Hazard ration | P-value | Hazard ration (95% CI) | P-value | Hazard ration (95% CI) | P-value | ||
| Univariate analysis | |||||||
| BMI: continuousb | 0.99 (0.97–1.02) | 0.59 | 1.01 (0.98–1.04) | 0.58 | 1.02 (0.98–1.06) | 0.44 | |
| BMI groups | 0.67 | 0.08 | |||||
| Overweight versus normal weight | 0.85 (0.61–1.19) | 0.35 | 1.22 (0.76–1.97) | 0.41 | 0.57 (0.33–0.98) | 0.04 | |
| Obese versus normal weight | 0.89 (0.64–1.26) | 0.53 | 1.01 (0.61–1.07) | 0.96 | 0.95 (0.58–1.56) | 0.84 | |
| Multivariable analysis | |||||||
| BMI:continuousb | 1.00 (0.97–1.03) | 0.96 | 1.00 (0.97–1.04) | 0.77 | 1.03 (0.98–1.08) | 0.26 | |
| BMI groups | 0.80 | 0.32 | |||||
| Overweight versus normal weight | 0.89 (0.63–1.26) | 0.52 | 1.09 (0.66–1.78) | 0.74 | 0.71 (0.37–1.33) | 0.28 | |
| Obese versus normal weight | 1.00 (0.69–1.41) | 0.96 | 0.92 (0.55–1.53) | 0.74 | 1.37 (0.74–2.54) | 0.31 | |
| List of factors adjusted | Age, sex, IPI, RCHOP versus CHOP, B-symptom, Sex:(RCHOP versus CHOP) | Age, sex, ABVD/Stanford V, IPS, B-symptom | Age, sex, B-symptom, FLIPI, maintenance treatment (no, R or OBS) | ||||
aBy weighted Cox regression model.
bCases with extreme BMI (BMI > 50) were removed.
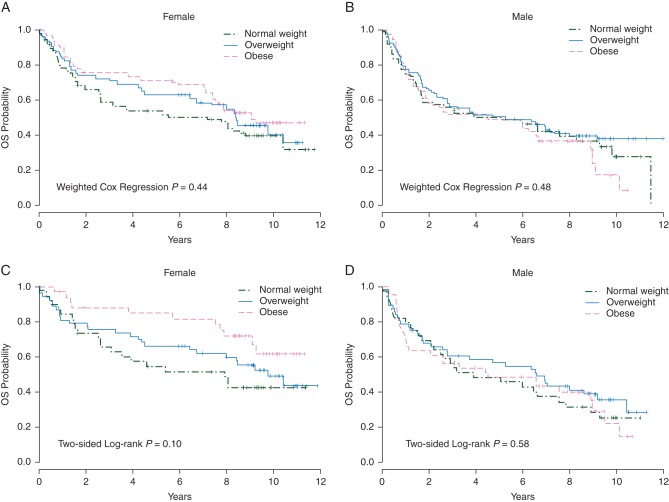
The separate analysis within each sex (Figure 2A and B) did not suggest any significant association of BMI with OS for either females or males. The subset analysis within patients treated with RCHOP (Figure 2C and D) suggested a potential trend of better survival probability with higher BMI among female patients (P = 0.10), but not among male patients (P = 0.58).
Figure 2.
Overall survival (OS) by BMI groups, for female patients (A) and male patients (B) stratified on treatment arms, female patients (C) and male patients (D) treated on RCHOP, in the E4494 trial.
Hodgkin's lymphoma
Among the total of 739 HL patients, 27% were overweight and 29% were obese. Age was significantly higher (P = 0.005) with higher BMI groups. Higher BMI groups have better performance status (P = 0.01), less frequent B-symptoms (P = 0.04) and less bone marrow involvement (P = 0.07). Other baseline factors are balanced, and actual treatment received was similar among BMI groups (supplementary Table S2, available at Annals of Oncology online). With a median follow-up of 6.4 years, there is no significant association between BMI with FFS (P = 0.30) or with OS (P = 0.67). The hazard ratio of OS was 1.22 (95% CI, 0.76–1.97) for overweight, and 1.01 (95% CI, 0.61–1.07) for obese, when compared with normal weight. After adjusting for known prognostic factors, BMI groups remained unassociated (P = 0.80) with OS outcome (Table 1). Continuous BMI is not significantly associated with OS when used alone (P = 0.58) or after adjusting for known prognostic factors in the multivariable Cox model (P = 0.77, Table 1).
follicular lymphoma
Among the total of 279 FL patients, 38% were overweight and 37% were obese. The overweight group was significantly younger (P = 0.03) than the normal weight and obese groups. Overweight and obese groups tended (P = 0.08) to have more male patients than normal weight groups. Other baseline factors were balanced, and actual treatment received was similar among BMI groups (supplementary Table S3, available at Annals of Oncology online). With a median follow-up of 8.5 years, there is no significantly association between BMI with FFS (P = 0.45). However, a trend toward better OS in the overweight group compared with the normal weight group, with 5-year OS of 73%, 80% and 77% for normal weight, overweight and obese groups, respectively (Figure 1). The hazard ratio of mortality was 0.57 (95% CI, 0.33–0.98) for overweight, and 0.95 (95% CI, 0.58–1.56) for obese, when compared with normal weight. However, the significant survival advantage of overweight group was lost (P = 0.28) adjusting for other baseline factors. Continuous BMI was not associated with OS (P = 0.44) in the univariate Cox model, and remained non-significant (P = 0.26) after adjusting for other baseline factors (Table 1).
As patients with missing FLIPI (n = 62, 22%) had more male, less B-symptom presentation, better performance status and less nodal groups compared with patients with FLIPI score, the results from multivariable analysis might not be applicable to the entire trial population. The subset analysis within n = 114 patients treated with MR suggested no significant association between BMI groups with FFS (P = 0.92) or with OS (P = 0.36).
sensitivity analysis
Data on B symptom (weight loss) were missing for 2, 25 and 0 cases from E4494, E2496 and E1496. Significant weight loss was reported in 89 (17%), 136 (18%) and 29 (10%) in the above three studies. Sensitivity analyses excluding those patients resulted in similar findings, indicating that BMI categories did not reach statistical significance, with P = 0.91, 0.09 and 0.70 for FFS, and P = 0.51, 0.16 and 0.18 for OS among patients with DLBCL, HL and FL, respectively.
discussion
Using the cohorts from three prospectively designed phase III trials, we failed to find significant associations between BMI and clinical outcomes both in terms of FFS and OS. Furthermore, the survival curves are rather overlapped among BMI groups for all three patient cohorts. The association remains non-significant after adjusting for important baseline prognostic factors, or limiting to patients who received rituximab treatment among patients with DLBCL or FL. The sensitivity analyses excluding patients with weight loss at baseline result in similar observations. When examining DLBCL patients within each sex and/or within RCHOP treatment, BMI was not shown to be significantly associated with survival among male patients with or without rituximab, but suggested a potential trend that higher BMI leads to longer survival among female patients treated with rituximab.
Landgren et al. [11] showed in an investigation of 301 HL patients that cause-specific survival was significantly better with higher BMI groups, before and after adjusting for other clinical factors. Treatment was based on BSA in both Landgren and E2496, suggesting that dosing is not a factor that contributes to the discrepant findings. The patients reported by Landgren et al. were diagnosed and treated between 1973 and 1994; while E2496 enrolled patients from 1999 to 2006. It is unclear whether the different treatment era contributes to the discrepant findings. Prognostic profiles (IPS, B-symptoms and Stage) were better for obese and overweight patients in Landgren; however, were not adjusted in the multivariable model.
Jones et al. [12] reported that improved survival is associated with increased BMI among 728 patients with DLBCL or grade 3 FL. However, the association is seen with OS and not with PFS, with a hazard ratio of 0.97 for continuous BMI with OS. They did not observe OS difference between overweight and obese groups, with a P-value of 0.052 between normal versus above-normal groups. After adjusting for known prognostic factors, the significant association only remains between the overweight group and all others (including normal and obese patents). However, a significantly improved OS was observed for higher BMI groups (P< 0.001) among over 2000 US veterans with DLBCL, and the significance remained after adjusting for baseline factors [13]. When limiting to only male patients with DLBCL under RCHOP treatment from E4494, our analysis shows no difference among BMI groups in terms of both PFS and OS. The reason for discrepancies in findings is not clear. In the report by Carson et al., three IPS factors (LDH, ECOG PS and number of extra nodal sites) were not collected and adjusted, and treatment plan was unknown.
It is generally believed that higher relative dose intensity and cumulative dosage, if tolerated, is likely associated with better outcome [18]. The standard treatment plan is to dose the patients based on BSA. Some believe this strategy leads to a relative ‘under-treatment’ for patients with normal or under-weight [11]. Others reported more frequent dose reduction observed in obese patients, which might actually receive lower relative chemotherapy doses [19]. Based on available data among HL patients from E2496, the rate of dose modification is not significantly higher in the obese group (82%) compared with that in normal (77%) and overweight groups (77%).
A previous study found that doxorubicin clearance was reduced in the obese [20] while another study observed this phenomenon in women but not men [21]. A recent report by Muller et al. [22] suggests that higher weight of males contribute to their faster rituximab clearance, thus they benefit less from the addition of rituximab. The pharmacokinetics differences between normal and overweight/obese people needs to be elucidated. Another area of consideration is the systemic and microenvironmental milieu in which tumors develop.
There are limitations in our analysis. Although the data were from prospectively designed trials, the trials were not designed to study the association of BMI with clinical outcome. All patients were treated according to the protocols; however, it does not necessarily reflect how they would be treated in usual care setting, especially for obese and overweight patients. Though we found no difference in cycles of treatment received among BMI groups, it would be desirable to study the actual dosage received.
The present analysis has several important strengths. First, we evaluated patients with three major lymphoma histologies. Second, we used data from prospectively designed phase III clinical trials with uniform data collection and disease assessment, which reduces unwanted data variation. Furthermore, the large sample sizes for each histology and long follow-up provide a reliable evaluation of the association. Finally, as all patients were treated on a clinical trial, weight/height, baseline prognostic factors and weight loses information were available for virtually all patients, reducing sample bias and enables further investigation of the association in the presence of other prognostic factors.
BMI is not associated with clinical outcome among patients with DLBCL, FL and HL, based on our three large phase III trials. The findings contradict some other previous reports on similar investigations. The reasons might be multi-fold and involve a complex interaction among treatment type, actual dosage intensity and pharmacokinetics differences. Further work will be required to understand the reasons for the observed discrepancies among studies, to elucidate the effect of BMI on treatment and survival.
funding
Funding support for FH is from ECOG statistical office and the Research Scientist Fund from the Department of Biostatistics and Computational Biology at Dana-Farber Cancer Institute.
disclosure
Consultant: LIG (Aura Sense); researching funding: BSK (Genentech); employment: SJH (Genentech); Stock: SJH (Roche). All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank patients and families for participating in the trials.
references
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG MD, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; 2008. [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Patel AV, Diver WR, Teras LR, et al. Body mass index, height and risk of lymphoid neoplasms in a large United States cohort. Leuk Lymphoma. 2013;54:1221–1227. doi: 10.3109/10428194.2012.742523. [DOI] [PubMed] [Google Scholar]
- 5.Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 6.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 7.Waalkes S, Merseburger AS, Kramer MW, et al. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 2010;21:1905–1910. doi: 10.1007/s10552-010-9618-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170:e75–e83. doi: 10.1016/j.jss.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar M, Nooka A, Langston A, et al. Impact of body mass index (BMI) on overall survival in myeloma. Proc Am Soc Hematol. 2012 abstr. 4289. [Google Scholar]
- 10.Tarella C, Caracciolo D, Gavarotti P, et al. Overweight as an adverse prognostic factor for non-Hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft. Bone Marrow Transplant. 2000;26:1185–1191. doi: 10.1038/sj.bmt.1702692. [DOI] [PubMed] [Google Scholar]
- 11.Landgren O, Andren H, Nilsson B, et al. Risk profile and outcome in Hodgkin’s lymphoma: is obesity beneficial? Ann Oncol. 2005;16:838–840. doi: 10.1093/annonc/mdi145. [DOI] [PubMed] [Google Scholar]
- 12.Jones JA, Fayad LE, Elting LS, et al. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:1649–1657. doi: 10.3109/10428194.2010.494315. [DOI] [PubMed] [Google Scholar]
- 13.Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3217–3222. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 15.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habermann TM, Hong F, Morrison VA. Differences in outcomes in males and females with diffuse large B-cell lymphoma with induction rituximab and in follicular lymphoma treated with maintenance rituximab. Proc Am Soc Hematol. 2012;3705 [Google Scholar]
- 18.Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87:277–283. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 19.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 20.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6:1321–1327. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 21.Sparreboom A, Wolff AC, Mathijssen RH, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25:4707–4713. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 22.Muller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–3284. doi: 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.