Figure 4.
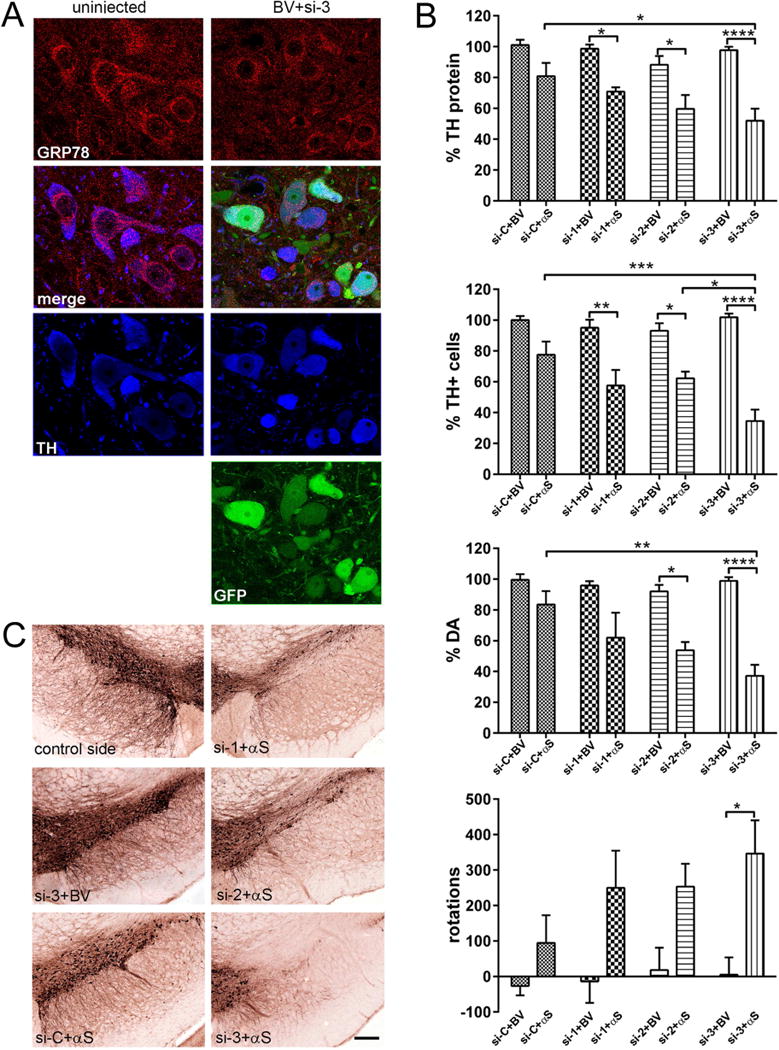
Degree of decline in GRP78 expression correlates with striatal TH protein levels, nigral DA cell survival and amplitude of behavioral asymmetry in response to human α-syn (αS) overexpression. (A) GRP78 protein expression level in DA neurons of the SNc at 3 months after rAAV-GRP78 shRNA injection. Confocal images show GRP78 (red) and TH (blue) as well as native GFP (green) in nigral neurons at 3 month after combined injection of viruses expressing si-3 and BV. TH is used as a marker for DA cells. Images illustrate protein expression levels of GRP78 (red) in nigral TH+ neurons (blue) on non-injected (left panel) and injected (right panel) sides. (B) The effect of GRP78 decline and human wt α-syn expression on striatal TH protein levels (upper graph), unbiased estimation of nigral TH+ neuron survival as well as striatal DA level and rotational behavior (bottom graph) at 3 months after rAAV injection. Striatal TH-protein level and the number of nigral TH+ cells remaining on the injected side shown as a percentage of the uninjected side ± SE. Amount of striatal TH protein, DA and number of nigral TH+ neurons were most dramatically reduced in si-3 + αS injected rats compared to control si-3 + BV as well as to si-C + αS (and si-2 + αS for TH cells count) injected animals. The amphetamine induced rotation test revealed a significant number of ipsilateral rotations only in rats injected with si-3 + αS versus control si-3 + BV animals. Two-way ANOVA analysis. Tukey’ multiple comparisons test are indicated as *, **, ***, **** = P < 0.05, 0.01, 0,001 and 0.0001, respectively vs. appropriate control rats; N = 6 (for control groups) and 10 (for experimental groups). (C) Bright-field photomicrographs showing remaining TH-positive cells in the SNc of representative animals from different experimental groups. Bar = 250 μm.
