Abstract
Chronic developmental lead exposure yielding very low blood lead burden is an unresolved child public health problem. Few studies have attempted to model neurobehavioral changes in young animals following very low level exposure, and studies are needed to identify tests that are sensitive to the neurobehavioral changes that may occur. Mechanisms of action are not yet known however results have suggested that hippocampus/dentate gyrus may be uniquely vulnerable to early chronic low-level lead exposure. This study examined the sensitivity of a novel odor recognition task to differences in pre-adolescent C57BL/6J mice chronically exposed from birth to PND 28, to 0 ppm (control), 30 ppm (low-dose), or 330 ppm (higher-dose) lead acetate (N = 33). Blood lead levels (BLLs) determined by ICP-MS ranged from 0.02 to 20.31 µg/dL. Generalized linear mixed model analyses with litter as a random effect showed a significant interaction of BLL × sex. As BLLs increased olfactory recognition memory decreased in males. Among females, non-linear effects were observed at lower but not higher levels of lead exposure. The novel odor detection task is sensitive to effects associated with early chronic low-level lead exposure in young C57BL/6J mice.
Keywords: developmental lead exposure, olfactory recognition memory, dentate gyrus, neurobehavioral toxicity
1. Introduction
The dangers of developmental lead exposure are well-documented and there is widespread recognition that even low-level exposure alters neurobehavior in young children. Child studies have suggested many neurobehavioral functions altered by early chronic low-level lead exposure. These include but are not limited to memory and learning, visual attention, abstract problem-solving, cognitive set-shifting, and motor dexterity (Bellinger and Needelman, 2003; Canfield et al., 2003; Franko et al., 2000; Gilbert and Weiss, 2006; Jusko et al., 2008; Landrigan et al., 2006; Lanphear et al., 1998; Lanphear et al., 2005; Needleman et al., 1990; Needleman et al., 1996; Schnaas et al., 2000; Sobin et al., 2015; Wasserman et al., 2000). The mechanisms by which low-level lead disrupts neurodevelopment however are not yet known and few animal models of early chronic low-level lead exposure have been proposed. In order to advance knowledge in this area, neurobehavioral tests that are sensitive to the effects of early chronic low-level lead exposure in animals are needed.
Of the neurocognitive disruptions identified in low-level lead-exposed children, changes in memory may have the most profound implications for life-long brain health. The brain regions critical for memory and learning, in particular the hippocampus/dentate gyrus regions, overlap neurogenesis pathways. Early disruption of these regions and pathways has the potential to alter neural pathway formation (Schafer et al., 2012), memory function, learning during development (Schinder and Gage, 2004) and neurogenesis during adulthood and aging (Jesseberg et al., 2009), perhaps increasing vulnerability to cognitive decline and dementia.
Only a few past studies have examined memory in rodents with early chronic low-level lead exposure. For example, in adulthood, BALB/c mice chronically exposed to 20 ppm lead acetate delivered in dam’s drinking water had diminished memory (object recognition memory task) as compared to controls (Azzaoui et al., 2009). In a similar study, as compared to controls, adult Wistar rats chronically exposed to 20 ppm of lead in dam’s drinking water had diminished spatial memory (water maze) (Kasten-Jolly et al., 2012) (blood lead levels were not reported). A recent study in our laboratory of pre-adolescent C57BL/6J mice showed that as blood lead level (BLL) increased, exploration of a novel environment decreased (Flores-Montoya and Sobin, 2014).
In the current study, the sensitivity of a novel odor detection paradigm to the effects of early chronic low-level lead exposure was examined in pre-adolescent C57BL/6J mice. It was hypothesized that from lowest to highest levels of lead exposure, as BLL increases, olfactory recognition memory decreases.
2. Method
2.1. Animals
All animal procedures had prior approval of the Institutional Animal Care and Use Committee (IACUC) and were carried out in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Research Council, 2011). C57BL/6J mice were purchased from Jacksons Laboratories and housed in the Bioscience Research Facility at the University of Texas at El Paso (UTEP). Mice were group housed by sex in ventilated cages (22.22 cm × 36.83 cm × 13.97 cm) with ad libitum access to food and water. The animal holding room had a temperature of 20°–26° C, relative humidity of 30 to 70 percent, and a 12 hour light-dark cycle.
Dams were mated beginning at post-natal day (PND) 40 using harem breeding. Two females were placed with one male, checked daily, and housed separately after vaginal plug was identified. Ten dams were mated with five sires. Nine of ten dams were successfully impregnated. Gestation durations were between 19 and 21 days. Prior studies suggested that early chronic low-level lead exposure may alter stress-responsive neuroimmune processes (Sobin et al., 2013) thus, to avoid stressing dams and pups, unculled litters were planned with sex and litter (as a random effect) controlled in all analyses. Seven dams produced litters ranging in size from 3 to 6 pups, N = 33, including 13 females and 20 males. Two remaining litters of one pup each were not included. Each litter was assigned to one of three lead treatments, either 0 ppm, control (n=10, 2 females; 8 males), 30 ppm, low-dose (n=10, 5 females and 5 males), and 330 ppm, higher-dose (n=13, 6 females and 7 males). No animals died during the course of the study.
2.2. Lead Exposure
Pups were exposed to lead via dams’ milk. From PND 0 to PND 28 dams were given either lead-treated water (30 ppm or 330 ppm 99.4% lead acetate crystals, Sigma Aldrich, St. Louis, MO) or sodium-treated water (30 ppm).
2.3. Behavioral Testing
Recognition memory was tested at PND 28 with a novel odor recognition (NODR) task. The protocol was based on those used in previously published protocols (Bevins and Besheer, 2006; “Simple Odor Recognition Protocol,” 2011). This task was adapted from a novel object recognition memory task (NOR task) (Bevins and Besheer, 2006). The original task included a training phase and a testing phase. During the training phase, mice were placed in a square arena and allowed to explore two identical objects located in the upper corners of the arena. The testing phase then follows an inter-trial interval (ITI). A familiar object was replaced with a novel object. Mice were returned to the arena and allowed to freely explore the familiar and novel objects. Mice with intact memory spend more time exploring the novel as compared to the familiar object. For the current study, odors rather than objects were used to maximize possible group differences. The odors selected were those published in previous mouse behavioral protocols (“Simple Odor Recognition Protocol,” 2011).
All testing occurred between 10:00 a.m. and 1:00 p.m. Three identical square Plexiglas arenas (8 in × 8 in × 24 in) equipped with a timer were used for habituation (10 min), training (10 min), and testing (5 min) phases, with 5 min inter-trial intervals (ITI) between each phase. During the ITI, mice were returned to a holding cage with home bedding.
For the habituation phase, animals were placed in the empty arena and allowed to freely explore. For the training phase, animals were placed in the second arena with two identically scented vehicles. Orange or almond food-grade edible natural liquid flavors (McCormic®) were sprayed on 1” mouse-shaped felt objects positioned in the upper left and right arena corners approximately 4 cm from each wall. For the testing phase, the familiar scented object was replaced with a novel (orange or almond) scented object. Fixed visual cues in the testing room external to the testing arena were asymmetrical and to accommodate this, the location of the novel odor was fixed to the upper right corner; “familiar” and “novel” orange or almond odors were counterbalanced. All arenas were cleaned with 10% isopropyl alcohol after each trial. Each mouse was returned to the home cage when testing was completed.
Video cameras placed over the top of the arenas recorded all mouse activity during testing. Video recordings were later scored by four raters trained to reliability and blind to experimental condition. Exploration was recorded when the mouse nose was oriented towards and within a 2 cm proximity to the odor vehicle. Inter-rater reliability was determined after rater training and during and after test scoring. All post-training and scoring reliabilities exceeded 0.90.
2.4. Blood Collection
Immediately after behavioral testing, mice were anesthetized with Avertin (5–10 ml). Animals were sexed and weighed after they were unresponsive to corneal touch and paw pinch tests. Heart blood was extracted (50 µL of blood per animal) via syringe puncture at the heart apex. Blood samples were refrigerated and processed for inductively coupled plasma mass spectrometry (ICP-MS) analysis within 72 hours of sample collection.
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis of Blood Lead
The detailed method for the measurement of BLL was previously described (Sobin et al., 2011). Briefly, an Agilent 7500ce ICP-MS with an octopole reaction system and a CETAC ASX-520 autosampler was used. A Micro Mist U-series nebulizer and a double-pass quartz spray chamber were used to introduce the samples into plasma. The instrument parameters were a carrier gas of 0.78 L/min, makeup gas of 0.15 L/min, RF power of 1420 W, and a spray chamber that was set to a temperature of 2°C. Samples were processed by filling a propylene tube with 5.58 mL of water, 300 µL of blood, 60 µL of aqueous internal standard solution, and 60 µL of aqueous 10 ppm gold in 3% hydrochloric acid solution. The samples were vortexed, and centrifuged for one minute at 2000 rcf and the supernatant was analyzed by ICP-MS. BLLs were determined in micrograms per deciliter (µg/dL).
2.6. Statistical Analyses
Data were entered, checked for accuracy, and examined for missing values, distribution properties, and outliers. No values were out of the range of plausible responses and all data were included for analyses. Data were analyzed with SAS Version 9.3. (SAS Institute Inc., Cary, North Carolina).
Preliminary analyses were conducted to determine whether exposure paradigm yielded differences between groups in blood lead and whether body weight was influenced by lead exposure. In the first preliminary analysis, BLL was predicted from group, sex, and group × sex, with litter included as a random effect. In the second preliminary analysis, body weight was predicted from BLL, sex and BLL × sex, with litter included as a random effect.
Primary analyses included tests of the main hypothesis and confirmatory tests of effects. The primary hypothesis for the study stated that, controlling for sex and litter, BLL predicted testing phase discrimination ratio such that as BLL increased the testing phase discrimination ratio (odor recognition memory) decreased. For confirmatory analyses, three additional models were tested to rule out the possibility that lead exposure induced differences that altered ambulatory exploration and thus the acquisition and/or recognition of odor information. In these models, associations were tested between BLL and training phase total exploration time, BLL and testing phase total exploration time, and BLL and training phase discrimination ratio (controlling for sex with litter as a random effect in all models.)
An effect of BLL on total training phase and/or total testing phase exploration time would indicate that lead exposure somehow impacted ambulatory exploration required for information acquisition during the training or testing phase of the task, respectively. An effect of BLL on the training phase discrimination ratio would indicate that BLL somehow predisposed the animal to exploration of a left or right spatial location (of identical odors), perhaps biasing acquisition of information for one vs. the other spatial location.
Total exploration times for the training phase and the testing phase represented odor exploration according to the criteria stated in 2.3 above. Training phase discrimination ratio was calculated by dividing the time spent exploring the upper right odor by the total time spent exploring both odors [Tright/(Tright+Tleft)]. Testing phase discrimination ratio was calculated by dividing the time spent exploring the novel odor by the total time exploring the novel and familiar odors [Tnovel/(Tnovel+Tfamiliar)] (Bevins and Besheer, 2006).
Generalized linear mixed model analyses with maximum likelihood estimates (GLIMMIX procedure) were used for the preliminary and main analyses. Fixed effects for the preliminary analysis predicting BLL included group, sex and the interaction group × sex, with litter included as a random effect. Fixed effects for the preliminary analysis predicting body weight included BLL, sex and BLL × sex. Fixed effects for the four primary analyses predicting task behavioral outcomes included BLL, sex, and the interaction BLL × sex, with litter included as a random effect in all models. The Gaussian distribution with an identity link function was specified and all models were checked for convergence and the G matrix estimate. Model significance was evaluated by examining fixed effect Type III F-values and significance for one main effect (BLL) and one interaction (BLL × sex) controlling for litter. Parameter estimate significance values indicated difference from zero for continuous variables (i.e., BLL) or a significant difference between groups for the parameter estimates of the categorical variable (i.e., sex).
Significance for the Type III fixed effect tested whether the variable or interaction estimate differed significantly from zero and indicated the amount of model variance accounted for by a given (continuous or categorical) variable or interaction. When the fixed effect F-value was statistically significant, relevant post-hoc tests of least square means (for possible categorical effects) were evaluated; or regression coefficients (for the significant continuous predictor BLL or interaction) were determined and tested. Post-hoc comparisons of least square mean differences for categorical (sex) effects were calculated using the Tukey-Kramer adjustment for multiple comparisons. Least square means (LSM) reflected the mean of a variable after co-varying other model factors, i.e., BLL and litter. Adjusted alpha < 0.05, and adjusted lower and upper 95% confidence intervals were used to evaluate all post-hoc comparisons.
3. Results
3.1. Subjects
All animals completed testing. No animals died during the study. No adverse physical or behavioral effects were observed in the lead exposed or control animals. Table 1 shows the means and standard deviations (SD) of BLL and body weight for males and females by exposure group. BLLs ranged from 0.02 to 20.31 µg/dL.
Table 1.
Mean and SD of blood lead level and body weight for males and females in each exposure group following early chronic lead exposure, N = 33.
| Lead Exposure Group | Blood Lead Levels (µg/dL)* | Body Weight (grams)* | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 0 ppm | 0.31 ± 0.02 | 0.02 ± 0.00 | 15.14 ± 1.09 | 14.33 ± 0.15 |
| 30 ppm | 3.10 ± 0.57 | 2.63 ± 0.25 | 12.81 ± 0.90 | 11.82 ± 0.91 |
| 330 ppm | 15.21 ± 3.86 | 12.92 ± 1.00 | 14.27 ± 1.59 | 12.46 ± 1.85 |
In mixed model analyses with litter included as a random effect, BLL was predicted by exposure group, and not by sex or the interaction of group × sex; body weight was marginally predicted by sex (t = 1.97, p = .06), and not by BLL or the interaction of BLL × sex.
The first preliminary analysis predicted BLL from group, sex and group × sex interaction, with litter included as a random effect. Only group was a significant predictor of BLL (t2/26 = 158.4, p < .01). BLL was not predicted by sex (t2/26 = 1.62, p = n.s.) or the interaction group × sex (t2/26 = 1.04, p = n.s.).
The second preliminary analysis predicted body weight from BLL, sex and the interaction, with litter included as a random effect. Only sex approached significance with males weighing more than females (t2/26 = 1.97, p = .06) (LSmeans (SE) males = 14.23 (0.35), females = 12.52 (0.43)). BLL and the interaction of BLL × sex did not contribute to body weight variability.
3.2. Novel Odor Recognition Task
The means and SDs for the training and testing phases of the task are shown in Table 2. Odor exploration times during the training phase (10 minute exploration) were of longer duration than those observed during the testing phase (5 minute exploration).
Table 2.
Exploration times (s) during training and testing phases of the novel odor recognition task in C57BL/6J mice at pre-adolescence, N = 33.
| Mean +SD | ||
|---|---|---|
| Training Phase | ||
| (Two identical odors placed equidistant from upper left and right corners of arena) | ||
| Left odor exploration | 13.8 + 9.4 | |
| Right odor exploration | 17.1 +13.6 | |
| Odor exploration training total | 30.9 +22.1 | |
| Testing Phase | ||
| (Left/familiar odor remains and the right familiar odor is replaced by the novel odor*) | ||
| Left/familiar odor exploration | 3.4 +2.9 | |
| Right/novel odor exploration | 4.2 +3.7 | |
| Odor exploration testing total | 7.6 +5.2 | |
Familiar and novel odors were counterbalanced (half of the animals in each exposure group had orange as the familiar odor and almond as the novel odor; half had almond as the familiar odor and orange as the novel odor).
3.2.1. Primary Analysis
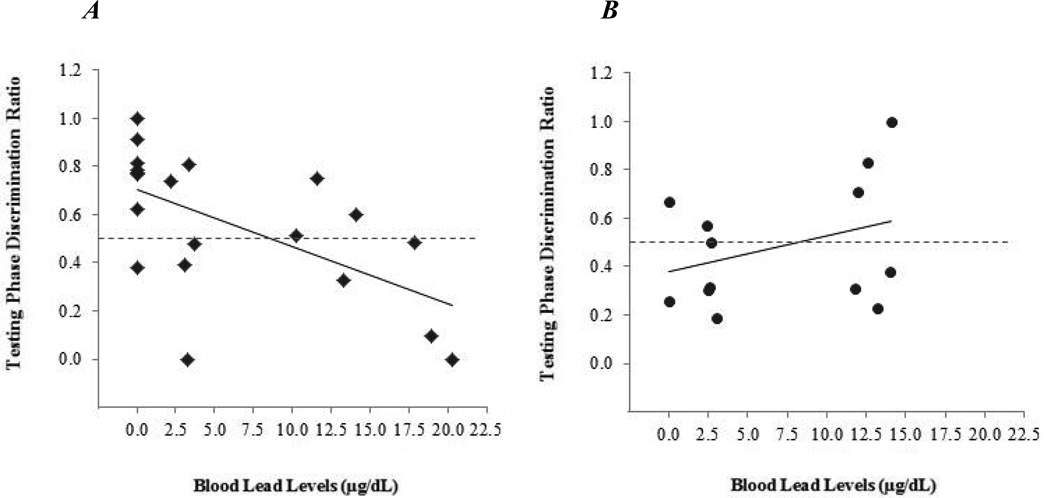
The primary model predicting testing phase discrimination ratio from BLL and sex controlling for litter was tested. Convergence criterion for the model was reached. The Type III fixed effect solutions and parameter estimates for the main effects of BLL and sex, and the interaction of BLL × sex are shown in Table 3. Only a significant interaction effect of BLL × sex was observed for the novel odor recognition testing phase. Tests of the regression coefficients for males (est = −0.024, SE = 0.01, df = 28, t = −3.06, p < .01) and for females (est = 0.01, SE = 0.01, df = 28, t = 1.22, p = 0.23) showed that the effect was significant only for males. Among males, for every 1 unit increase in BLL, testing phase discrimination ratio decreased by 0.024. The effects in males and in females are separately illustrated in Figure 1, A and B.
Table 3.
Parameter estimates showing effects of BLL, sex, and BLL × sex on novel odor exploration in C57BL/6J mice, controlling for litter (as a random effect).
| Significant Type III Fixed Effect | Solutions for Fixed Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | Est | SE | DF | t value | p | |||
| Training Phase | Intercept* | 0.50 | 0.03 | 28 | 15.96 | <0.01 | |||
| Discrimination Ratio | |||||||||
| BLL | 1.31 | 0.26 | BLL | 0.01 | 0.01 | 28 | 1.13 | 0.27 | |
| Sex | 3.50 | 0.07 | Sex male | 0.08 | 0.04 | 28 | 1.87 | 0.07 | |
| BLL × Sex | 0.57 | 0.46 | Sex female | 0 | . | . | . | . | |
| BLL × Sex male interaction | −0.01 | 0.01 | 28 | −0.75 | 0.48 | ||||
| BLL × Sex female interaction | 0 | . | . | . | |||||
| Testing Phase | Intercept* | 0.51 | 0.07 | 28 | 7.48 | <0.01 | |||
| Discrimination Ratio | |||||||||
| BLL | 0.37 | 0.58 | BLL | 0.01 | 0.01 | 28 | 0.58 | 0.23 | |
| Sex | 1.04 | 0.32 | Sex male | 0.05 | 0.09 | 28 | 0.62 | 0.32 | |
| BLL × Sex | 7.16 | 0.01 | Sex female | 0 | . | . | . | . | |
| BLL × Sex male interaction | −0.04 | 0.01 | 28 | −2.68 | 0.01 | ||||
| BLL × Sex female interaction | 0 | . | . | . | . | ||||
| Training Phase | Intercept* | 42.2 | 13.97 | 28 | 3.17 | <0.01 | |||
| Total Odor Exploration (s) | 0 | ||||||||
| BLL | 0.45 | 0.51 | BLL | 1.57 | 1.37 | 28 | 1.41 | 0.26 | |
| Sex | 0.20 | 0.65 | Sex male | −3.65 | 8.07 | 28 | −0.69 | 0.65 | |
| BLL × Sex | 1.74 | 0.20 | Sex female | 0 | . | . | . | . | |
| BLL × Sex male interaction | −1.69 | 1.28 | 28 | −1.36 | 0.20 | ||||
| BLL × Sex female interaction | 0 | . | . | . | . | ||||
| Testing Phase | Intercept* | 7.25 | 1.51 | 28 | 4.81 | <0.01 | |||
| Total Odor Exploration (s) | |||||||||
| BLL | 0.11 | 0.74 | BLL | 0.01 | 0.27 | 28 | 0.02 | 0.99 | |
| Sex | 0.07 | 0.80 | Sex male | 0.50 | 1.93 | 28 | 0.26 | 0.80 | |
| BLL × Sex | 0.13 | 0.72 | Sex female | 0 | . | . | . | . | |
| BLL × Sex male interaction | −0.11 | 0.32 | 28 | −0.37 | 0.72 | ||||
| BLL × Sex female interaction | 0 | . | . | . | . | ||||
BLL values were centered for these analyses and the intercepts are directly interpretable.
Figure 1.
Regression lines showing the association between blood lead level and test phase discrimination ratio for males (Figure 1A) and females (Figure 1B).
The y-axis shows the amount of time subjects explored the novel as compared to novel and familiar odors (discrimination ratio). Males (A) and females (B) are graphed separately. Subjects above the dashed line explored the novel odor for more time than the familiar odor; subjects below the dashed line explored the familiar odor for more time than the novel odor. A linear effect of BLL on discrimination ratio was found only for males. Among females, a linear effect was not found, however at lowest levels of exposure 5 of 7 female subjects performed at or below chance. The dashed lines shown on the graphs indicate equal amounts of time spent exploring the novel and familiar odor.
3.2.2. Confirmatory Analyses
Three additional models were calculated to test whether lead exposure had altered exploratory ambulation in males and/or females. If so, these possible effects would have been expected to alter information acquisition during the learning phases, and thus confound the primary outcome. The three confirmatory models tested whether BLL, sex and/or BLL × sex, with litter included as a random effect, predicted total exploration time during the training phase, total exploration time during the testing phase, and/or training phase discrimination ratio. As shown in Table 3, neither BLL nor the interaction of BLL × sex were predictive of total exploration time during the training phase, total exploration during the testing phase, or training phase discrimination ratio (calculated according to the formula provided in section 2.6.)
4. Discussion
The NODR task was sensitive to the linear effects of chronic developmental lead exposure on olfactory memory in young C57BL/6J mice. In males, as BLL increased, the amount of time spent exploring the novel as compared with the familiar odor decreased. Effects among females were suggested only at lowest levels of exposure (Figure 1) but were not linear and were not statistically significant in the tests conducted. (Results in females are discussed below.)
Broadly consistent with these findings, previous studies of adult mice with chronic lead exposure yielding low BLLs (discussed in 1. Introduction) reported deficits in object recognition (Azzaoui et al., 2009) and in spatial memory (Kasten-Jolly et al., 2012), similar to those found in the current study. Object recognition and spatial memory are sub-served by brain regions that overlap olfactory recognition memory pathways including the prefrontal, entorhinal and perirhinal cortices, and the hippocampus/dentate gyrus (Jesseberger et al., 2009; Parron et al., 2006; Rinaldi et al., 2007; Winters and Bussey, 2005). Given the current findings, future studies could examine whether object recognition and spatial memory deficits also emerge before adulthood in pre-adolescent mice with early chronic low-level lead exposure.
This study included confirmatory analyses to test whether the effects of BLL on exploratory ambulation may have accounted for the association between BLL and testing phase discrimination ratio. This is an important step for the interpretation of findings. Apparent effects on memory might be accounted for by differences in, for example, lead-induced deficits in motor function. In this study, no additional effects were identified. These tests provided some assurance that any apparent effects of BLL on the testing phase discrimination ratio would not be attributable to lead-induced differences in “familiar” odor exploration time, odor location preference, or differences in the patterns of left-right exploration. Furthermore, the validity of the NODR task was suggested by a significant increase in the discrimination ratio during the testing phase as compared with the training phase among control animals. In other words, control animals remembered the familiar odor and recognized the novel odor. This provided evidence that the NODR task is a valid test of olfactory recognition memory in young C57BL/6J mice.
Among females at lowest levels of exposure, memory deficits were perhaps apparent, i.e., the majority of the females did not discriminate the familiar from the novel odor. At the same time, performance among low-level lead exposed females did not appear to be linearly related to blood lead. It is possible that the limited variability in BLL at lower levels of exposure limited the detection of a linear effect among females. To address this issue, future studies examining effects on memory of early chronic low-level lead exposure should include a moderate exposure group to achieve a broader distribution of BLLs from lowest to higher values. Among other things, these findings suggested that the effects of lower and higher levels of lead exposure may be qualitatively different among males and females; and further, that future studies should be designed to ensure that this interaction is characterized.
Another methodological issue concerned overall exploration times. The testing phase exploration times were notably shorter than the exploration times recorded during the training phase and a restricted range of values may limit the detection of linear effects. For future studies, modifications to the protocol here described could include longer ITI periods which may result in increased exploration times during the testing phase of the task.
In this study, linear regression analyses suggested that among males, as BLLs increased, males spent greater amounts of time exploring the familiar as compared with the novel odor. An obvious conclusion is that lead exposure impaired the ability of mice to remember the familiar odor, resulting in continued exploration of the familiar odor rather than the novel odor. It was noted however that among lead exposed mice, testing phase discrimination ratios did not cluster near 0.50 but instead dropped to lower levels suggesting perhaps a preference for the familiar odor. Thus, another interpretation may be suggested. Past studies have suggested that exploratory behavior represents the predominance of curiosity over fear (Hughes, 2007). It is possible that the effects of lead exposure on brain resulted in decreased curiosity and/or increased fear of the novel odor. Future behavioral studies could use tests that separately challenge fear and curiosity in non-memory tasks, to determine whether low-level lead exposure disrupts learning by altering the balance of fear and curiosity.
The results of the present study suggested that chronic low-level lead exposure diminished olfactory recognition memory in pre-adolescent male mice. For future mechanistic studies, it may be useful to briefly consider possible molecular pathways underlying these observed effects. The ability of mice to detect and remember novel and familiar odors is dependent on the integrity of olfactory recognition memory pathways. When healthy mice are exposed to a novel or familiar odor, G-protein coupled receptors located in the epithelium of the nose detect odors and transmit information to the main olfactory bulb (Imai et al., 2006). The olfactory bulb has major projections to the piriform cortex which in turn projects to the thalamus, orbitofrontal cortex, hypothalamus, entorhinal cortex, and hippocampus (Sanchez-Andrade et al., 2005) ultimately allowing the detection, storage, and retrieval of olfactory information. Future studies could investigate whether low-level lead exposure disrupts odor detection, storage and/or retrieval by impairing the transmission of olfactory information via one or more of these pathways.
4.1. Strengths and Limitations
The present study is the first to test memory in pre-adolescent mice with chronic low-level lead exposure, and is one of few animal studies that examined effects on cognitive behavior following early chronic low-level lead exposure in animals with BLLs < 5 µg/dL. These animal studies were an extension of the current child literature showing memory deficits in pre-adolescent children with BLLs < 5 µg/dL (Chiodo et al., 2004; Chiodo et al., 2007; Min et al., 2007; Sobin et al., 2015; Surkan et al., 2007) and suggested that a C57BL/6J mouse model of early chronic low-level lead exposure can be useful for understanding neurobehavioral effects in young human populations. The relatively short exploration times during the testing phase and the unbalanced numbers of males and females were weaknesses in the present study and the results may not fully characterize sex differences across groups.
5. Conclusion
The NODR task detects memory deficits associated with early chronic low-level lead exposure in pre-adolescent C57BL/6J male mice. Future studies could use a battery of tasks that attempt to differentiate deficits in fear and/or curiosity that may contribute to the observed effects on odor recognition memory, and that assesses and compares deficits in recognition memory for odors, objects, and spatial location. The findings may suggest novel mechanisms of action for future molecular studies.
Highlights.
Olfactory memory was examined in mice with and without early chronic lead exposure.
Blood lead levels in exposed mice were 2.02 to 20.3 micrograms per deciliter.
In males, as blood lead level increased olfactory memory decreased.
In females, a non-linear effect was observed at lowest levels of exposure.
Acknowledgments
The authors would like to acknowledge Mari Golub, Environmental Toxicology, UC Davis, for her assistance in the preparation of the final manuscript. This research was made possible by grants from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, (R21HD060120, CS, PI); the National Center for Research Resources, a component of the National Institutes of Health (5G12RR008124); the Center for Clinical and Translational Science, The Rockefeller University, New York, New York; the Paso del Norte Health Foundation, El Paso, Texas; and from funding sources at the University of Texas, El Paso including, the Border Biomedical Research Center (BBRC); the University Research Institute (URI); and by funds provided from the J. Edward and Helen M.C. Stern Endowed Professorship in Neuroscience (CS). The funders had no role in the design, collection, data analyses and interpretation, implementation, manuscript preparation for this study, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azzaoui FZ, Ahami AO, Khadmaoui A. Impact of lead sub-chronic toxicity on recognition memory and motor activity of Wistar rat. Pakistan Journal of Biological Science. 2009;12:173–177. doi: 10.3923/pjbs.2009.173.177. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Needleman HL. Intellectual impairment and blood lead levels. New England Journal of Medicine. 2003;349:500–502. doi: 10.1056/NEJM200307313490515. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial nonmatching to-sample learning task to study ‘recognition memory.’. Nature Protocols. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 micrograms per deciliter. New England Journal of Medicine. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicology and Teratology. 2004;26:359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J, Delaney Black V. Blood lead levels and specific attention effects in young children. Neurotoxicology and Teratology. 2007;29:538–546. doi: 10.1016/j.ntt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Flores-Montoya MG, Sobin C. Early chronic lead exposure reduces exploratory activity in young C57BL/6J mice. Journal of Applied Toxicology. 2014;12 doi: 10.1002/jat.3064. (epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko E, Palome J, Brown M, Kennedy C, Moore L. Blood lead levels in young children-United States and selected states, 1996–1999. Morbidity and Mortality Weekly Report. 2000;49:1133–1137. [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to [mu]g/dL. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Neotic preferences in laboratory rodents: issues, assessment and substrates. Neuroscience and Biobehavioral Reviews. 2007;31:441–464. doi: 10.1016/j.neubiorev.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314:657–661. doi: 10.1126/science.1131794. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning & Memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 mg/dL and child intelligence at 6 years of age. Environ Health Perspectives. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten-Jolly J, Pabello N, Bolivar VJ, Lawrence DA. Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology. 2012;33:1005–1020. doi: 10.1016/j.neuro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Trasande L, Thorpe LE, Gwynn C, Lioy PG, Lipkind HS, Susser E. The national children's study: A 21-year prospective study of 100 000 american children. Pediatrics. 2006;118:2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Burgoon DA, Rust SW, Eberly S, Galke W. Environmental exposures to lead and urban children's blood lead levels. Environmental Research. 1998;76:120–130. doi: 10.1006/enrs.1997.3801. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Roberts R. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environmental Health Perspectives. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-Y, Min K-B, Cho S-I, Kim R, Sakong J, Paek D. Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology. 2007;28:421–425. doi: 10.1016/j.neuro.2006.03.007. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). Institute for Laboratory Animal Research (U.S.), & National Academies Press (U.S.) Guide for the care and use of laboratory animals. Washington, D.C: National Academies Press; 2011. [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. New England Journal of Medicine. 1990;322:83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275:363–369. [PubMed] [Google Scholar]
- Parron C, Save E. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiology of Learning and Memory. 2004;82:1–11. doi: 10.1016/j.nlm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology. 2007;32:309–319. doi: 10.1038/sj.npp.1301176. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. Journal of Reproduction and Development. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Schafer D, Lehrman E, Kautzman A, Koyama R, Mardinly A, Yamasaki R, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology. 2004;9:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Perroni E, Martinez S, Hernandez C, Hernandez RM. Temporal pattern in the effect of postnatal blood lead level on intellectual development of young children. Neurotoxicology and Teratology. 2000;22:805–810. doi: 10.1016/s0892-0362(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Simple Odor Recognition Protocol. [Retrieved on August 26, 2014];2011 from http://nbc.jhu.edu/behavioral_tasks/tasks/simple_odor_recognition_protocol.html. [Google Scholar]
- Sobin C, et al. A Bland-Altman comparison of the Lead Care(R) System and inductively coupled plasma mass spectrometry for detecting low-level lead in child whole blood samples. Journal of Medical Toxicology. 2011;7:24–32. doi: 10.1007/s13181-010-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Flores-Montoya MG, Gutierrez M, Parisi N, Schaub T. Δ Aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicology and Teratology. 2015;47:137–145. doi: 10.1016/j.ntt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 [mu]g/Dl. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman G, Liu X, Popovac D, Factor-Litvak P, Kline JK, Waternaux C, Graziano JH. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicology and Teratology. 2000;22:811–818. doi: 10.1016/s0892-0362(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]