Abstract
During metamorphosis the CNS undergoes profound changes to accommodate the switch from larval to adult behaviors. In Drosophila and other holometabolous insects, adult neurons differentiate either from respecified larval neurons, newly born neurons, or are born embryonically but remain developmentally arrested until differentiation during pupal life. This study addresses the latter in the identified Drosophila flight motoneuron 5. In situ patch-clamp recordings, intracellular dye fills and immunocytochemistry address the interplay between dendritic shape, excitability and ionic current development. During pupal life, changes in excitability and spike shape correspond to a stereotyped, progressive appearance of voltage-gated ion channels. High-voltage-activated calcium current is the first current to appear at pupal stage P4, prior to the onset of dendrite growth. This is followed by voltage-gated sodium as well as transient potassium channel expression, when first dendrites grow, and sodium-dependent action potentials can be evoked by somatic current injection. Sustained potassium current appears later than transient potassium current. During the early stages of rapid dendritic growth, sodium-dependent action potentials are broadened by a calcium component. Narrowing of spike shape coincides with sequential increases in transient and sustained potassium currents during stages when dendritic growth ceases. Targeted RNAi knockdown of pupal calcium current significantly reduces dendritic growth. These data indicate that the stereotyped sequential acquisition of different voltage-gated ion channels affects spike shape and excitability such that activity-dependent calcium influx serves as a partner of genetic programs during critical stages of motoneuron dendrite growth.
Keywords: differentiation, ion channel, ionic current, patch-clamp
Introduction
Differentiation of neurons comprises the generation of a neuron-type-specific ‘gestalt’ by growth and targeting of axon and dendrites, as well as the acquisition of specific sets of voltage- and ligand-gated ion channels that produce distinct excitabilities (Spitzer et al., 2002). Many of the underlying developmental mechanisms are controlled genetically, but it has become clear that activity is a necessary partner of almost all aspects of neuronal differentiation (Spitzer, 2006), especially via interactions of calcium influx with genetic programs (Niell et al., 2004; Van Aelst & Cline, 2004; Lohmann & Wong, 2005; Baines & Pym, 2006; Spitzer, 2012). Consequently, the development of a neuron’s excitability is directly related to other aspects of differentiation and, thus, should be studied in parallel to structural development.
Differentiation needs to be regulated correctly during embryonic development, but potentially also following injury, or even following adult neurogenesis (Nottebohm, 2002; Kempermann, 2012). A special case is the metamorphosis of amphibians (Kelley & Dennison, 1990) or holometabolous insects (Consoulas et al., 2000), during which the whole body is changed from a larval to an adult ‘bauplan’.
In insect metamorphosis three modes of neuronal differentiation have been described. First, many sensory neurons and interneurons are newly born and become integrated into adult-specific networks (Meinertzhagen, 1993). Second, many modulatory neurons and motoneurons are born embryonically to first serve a larval function and then become remodeled to accommodate new functions in the adult (Copenhaver & Truman, 1986; Duch et al., 2000). And third, some neurons are born embryonically but do not serve a larval function. Instead, these neurons remain undifferentiated until early pupal life to then differentiate into mature, adult-specific neurons. Examples of central neurons that stay developmentally arrested until they progressively acquire adult features during metamorphosis are the Drosophila melanogaster tergo-trochanteral jump motoneuron (Jacobs et al., 2000) and motoneuron 5 (MN5; Consoulas et al., 2002), one of five flight motoneurons innervating the dorsolongitudinal wing depressor muscle (DLM; Ikeda & Koenig, 1988).
MN5 is a large monopolar motoneuron located on the dorsal surface of the mesothoracic neuromere of the fruit fly’s ventral ganglion, innervating fibers 5 and 6 of the contralateral DLM. The postembryonic development of MN5 axon terminals (Fernandes & Keshishian, 1998), its complex dendritic arbor (Vonhoff & Duch, 2010) and neurotransmitter receptor expression (Kuehn & Duch, 2013) are described. It is also known that activity is a key player in MN5 structural differentiation (Vonhoff et al., 2013).
This study addresses the functional interplay between structural and physiological differentiation of developmentally arrested motoneurons during metamorphosis. We analyse the developmental progression of MN5 excitability and the order of expression of voltage-gated calcium (VGCCs), sodium (VGSCs) and potassium channels to then relate physiological development to the time course of MN5 dendrite growth. A stereotyped sequential acquisition of different voltage-gated ion currents underlies progressive changes in action potential shape, with a calcium-dependent spike component at stages of rapid dendritic growth. A functional role of VGCCs in dendritic growth is confirmed by targeted genetic manipulation.
Materials and methods
Animals
Drosophila melanogaster were reared at 25 °C at a 12 : 12 h light/dark cycle on a cornmeal-yeast-agar diet in Drosophila plastic fly vials with mite-proof foam stoppers (Dutscher Scientific UK). All animals used were F1 progeny from the following cross: female w; UAS-mCD8-GFP; D42-GAL4, Cha-GAL80 crossed to male w1118. Experiments were carried out using Drosophila pupae of different stages. Stage determination was carried out by external anatomical criteria according to Bainbridge & Bownes (1981). Briefly, Drosophila metamorphosis lasts approximately between 90 and 100 h, and is divided into 15 stages with varying duration. Some of the stages are divided into sub-stages.
Adult flies were dissected as previously described (Ryglewski et al., 2012). For pupal dissection the pupal case was carefully removed using forceps. The MN5 soma could readily be identified without GFP-expression from P4 to P13. Expression of UAS-mCD8-GFP under the control of D42-GAL4 (Yeh et al., 1995; Sanyal et al., 2003; Ryglewski et al., 2012) aided in determining the location of the MN5 soma within the dense mesothoracic neuropils of late-stage pupae.
Electrophysiology
In situ whole-cell patch-clamp experiments in current-clamp and voltage-clamp mode were carried out as described previously (Ryglewski et al., 2012). Briefly, after mounting the Petri dish with the dissected animal on an upright Zeiss Axio Examiner A1 fixed-stage microscope, the MN5 soma was focally cleaned under visual control using a 40 ×/1.0 DIC VIS-IR water dipping lens using 2% protease in saline in a patch pipette with a broken tip (Ryglewski & Duch, 2012). After cleaning the neuron, the preparation was rinsed for approximately 15 min with either normal saline or calcium current recording solution (see below; Ryglewski et al., 2012).
MN5 was approached using a patch pipette with a tip resistance of approximately 4–5 MΩ in normal saline in combination with normal intracellular recording solution (see below) pulled from borosilicate glass capillaries (o.d. 1.5 mm, i.d. 1 mm, no filament; World Precision Instruments, Sarasota, FL, USA). After seal formation and membrane rupture, recordings were allowed to stabilize for approximately 2 min before experiments were carried out. Intracellular patch solution differed depending on whether potassium currents and action potentials or calcium currents were recorded (for composition, see below; Ryglewski & Duch, 2009; Ryglewski et al., 2012). For calcium current measurement, voltage-gated potassium channels and VGSCs were blocked with 4-aminopyridine (4-AP), tetraethylammonium-chloride and -bromide (TEA-Cl, TEA-Br) or tetrodotoxin (TTX, 100 nM, applied directly into the bath), respectively. High-voltage-activated calcium currents were blocked by application of Plectreurys tristis toxin II (PLTXII, 20 nM; Branton et al., 1987). Access resistances were between 6 and 9 MΩ as read from the patch-clamp amplifier after compensation. Current run-down was not observed. Recordings were carried out with a steady perfusion to constantly provide fresh saline. Saline flow was only stopped for 2 min for TTX application (see below). TTX action was not reversed by subsequent perfusion with recording solution, whereas PLTXII action was. Therefore, calcium currents recorded directly prior to or right after PLTXII application were recorded with halted perfusion to prevent washout of the drug.
For outward potassium current measurements, sodium channels were blocked with TTX. We have not used calcium channel blockers, because we wanted to record total outward current consisting of sole voltage-dependent and calcium-dependent components (Ryglewski & Duch, 2009). Calcium currents can only be recorded from MN5 in the presence of extracellular potassium channel blockers and intracellular cesium. Strong outward current block and low access resistances (< 12 MΩ) are required to make the cell compact enough to record calcium currents (Ryglewski et al., 2012). All outward currents were recorded without cesium in the patch pipette, thus making an artificial reduction of outward current by inward calcium currents unlikely. However, we cannot exclude the possibility of some outward current contamination by residual inward current.
Data were recorded with an Axopatch 200B amplifier and digitized at 20 kHz using a Digidata 1440a (both Molecular Devices), and filtered through a 5-kHz low-pass Bessel filter. Data acquisition and analysis were carried out using pClamp 10.3 software (Molecular Devices) and Excel 2010 (Microsoft).
Action potentials were blocked with the VGSC blocker TTX (100 nM; Sigma, Germany) pipetted directly into the recording chamber, halting the perfusion system for 2 min for application only (see above).
Recording solutions (composition in mM)
Normal saline: NaCl, 128; KCl, 2; MgCl2, 4; CaCl2, 1.8; HEPES, 5; sucrose, ~35. Calcium current recording saline: NaCl, 93; KCl, 5; MgCl2, 4; CaCl2, 1.8; BaCl2, 1.8; TEA-Cl, 30; 4-AP, 2; HEPES, 5; sucrose, ~35. pH of both solutions was adjusted to 7.24 with NaOH, osmolality was adjusted to 290 mOsM/kg (normal saline) or 320 mOsM/kg (calcium current recording solution) with sucrose if necessary, and depended on the osmolality of the respective intracellular solution. For some experiments the calcium channel blocker cadmium was used (cadmium chloride, 500 μM) in the calcium current recording solution.
Normal intracellular recording solution (potassium current and action potential recordings): K-gluconate, 140; Mg-ATP, 2; MgCl2, 2; EGTA, 11; HEPES, 10. pH was adjusted to 7.24 with KOH, osmolality was adjusted to 300 mOsM/kg with glucose if necessary. Intracellular calcium current recording solution: CsCl, 140; CaCl2, 0.5; Mg-ATP, 2; EGTA, 11; TEA-Br, 20; 4-AP, 0.5; HEPES, 10. pH was adjusted to 7.24 with CsOH, osmolality was already at 323 mOsM/kg, and therefore it was not adjusted. All chemicals for saline and intracellular recording solutions were obtained from Sigma, Germany. PLTXII was obtained from Alomone Labs, Israel.
Current-clamp analysis
Five different electrophysiological parameters were determined by current-clamp recordings in normal extra- and intracellular solution (for composition, see above; Table 1): resting membrane potential in mV (Vrest); firing threshold in mV; time to peak in ms; amplitude in mV; and half-maximal amplitude width (50% ampl. width) at various different pupal stages. Vrest was obtained directly after break-in in current-clamp mode without holding current injection. Action potentials were evoked by somatic current injections. The firing threshold was measured at the steepest slope at action potential onset following current injections to just threshold levels (Fig. 2D, dashed line labeled threshold). Time to peak and amplitude were measured between threshold and action potential peak voltage (Fig. 2D). Half-amplitude width was defined as the action potential width at the voltage in the middle between threshold and maximum amplitude (Fig. 2D).
Table 1.
Action potential analysis in MN5
| Dev. stage | Vrest (mV) | Firing threshold (mV) | Time to peak (ms) | Amplitude (mV) | 50% ampl. width (ms) | n |
|---|---|---|---|---|---|---|
| P5 | −54.7 ± 8.8 | −1.5 ± 5.2 | 3.53 ± 1.61 | 7.1 ± 2.4 | 3.11 ± 0.70 | 12 (4) |
| P7 | −59.6 ± 2.6 | −12.0 ± 7.2 | 1.92 ± 0.19 | 21.1 ± 5.9 | 4.31 ± 0.23 | 3 (3) |
| P8 | −57.4 ± 4.0 | −21.5 ± 8.4 | 2.25 ± 0.34 | 23.9 ± 15.1 | 4.94 ± 1.52 | 14 (12) |
| P10 | −55.4 ± 4.0 | −25.3 ± 4.5 | 1.42 ± 0.26 | 37.0 ± 12.9 | 1.99 ± 0.39 | 6 (6) |
| P12 | −58.8 ± 5.2 | −20.4 ± 3.6 | 1.23 ± 0.07 | 43.7 ± 9.6 | 1.38 ± 0.06 | 7 (7) |
| P15 | −55.2 ± 2.0 | −24.3 ± 2.3 | 0.95 ± 0.20 | 44.7 ± 2.6 | 1.07 ± 0.28 | 4 (4) |
| Adult | −67.8 ± 5.5 | −24.1 ± 8.2 | 1.20 ± 0.33 | 45.5 ± 8.6 | 0.92 ± 0.25 | 13 (11) |
Values are mean ± SD. Values in parentheses are n’s for action potential analysis. Other n’s are values for Vrest only.
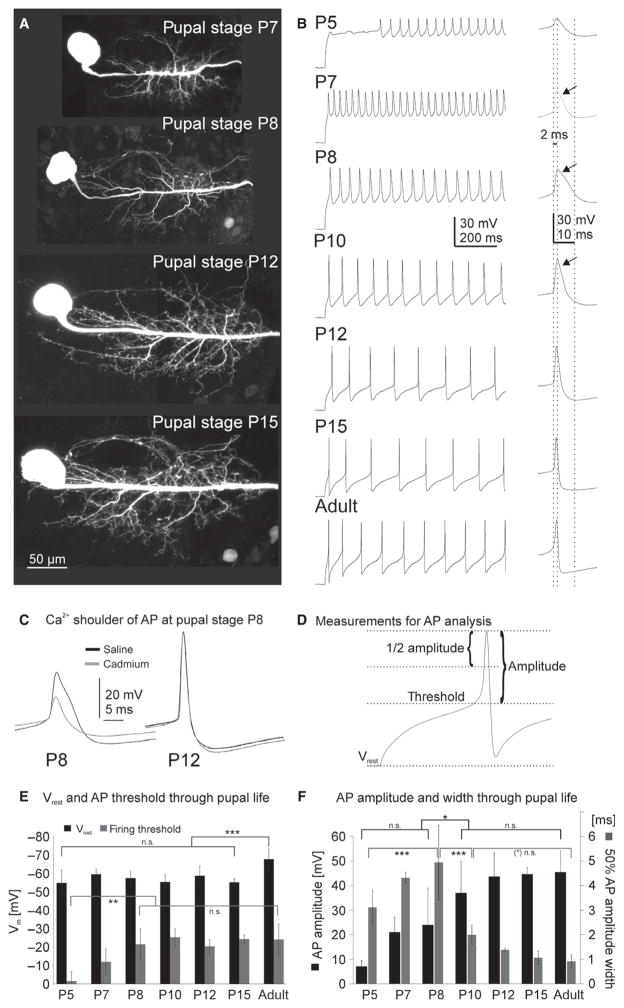
Fig. 2.
Differentiation of MN5 structure and excitability during metamorphosis. (A) Representative maximum projection views from confocal image stacks of intracellular dye fills of MN5 at different pupal stages. MN5 dendritic differentiation was well underway by pupal stage P7 (A, top image). Rapid dendrite growth occurred until pupal stage P12 (A, P8, P12), which was followed by moderate growth until the mature dendritic structure was present at P15 just prior to eclosion (A, bottom image). (B) MN5 firing responses to depolarizing somatic current injection through pupal life. The right panel in (B) depicts enlargements of single action potentials. Dotted lines align action potential onsets at different stages, and demark 2-ms and 10-ms time points after onset. At P5, a delay to the first spike was observed (B, top trace). First action potentials at P5 were of low amplitude (~20 mV) and broad (~10 ms from threshold to undershoot). The amplitude increased as metamorphosis continued (B). Action potentials at P7–P10 (second–fourth traces from top) exhibited a plateau-like shoulder (B, see arrows in enlargements) that was most pronounced at P8. This shoulder was calcium dependent (C, left trace), and was not observed from P12 on (C, right trace), when action potential width and amplitude were adult-like (B, third trace from bottom). Membrane potential and action potential analysis was based on measures as depicted in (D). The resting membrane potential was about −55 mV through all stages of pupal life, but significantly decreased in the adult MN5 (E, black bars). The firing threshold increased significantly during early pupal life until P8, and then remained unchanged until adulthood (E, gray bars). Action potential amplitudes increased significantly between P8 and P10 (F, black bars), and showed no significant increases from P10 on until adulthood. By contrast, action potential width at half-maximum amplitude increased significantly from P5 over P7 until P8 (F, gray bars), when the calcium-dependent shoulder was most pronounced (B). It then decreased significantly between P8 and P10 (F, gray bars), and further between P10 and P12 (F, gray bars). Errors represent SD. Data were analysed by ANOVA and LSD pair-wise post hoc comparisons. Statistically significant differences were assumed at *P < 0.05, **P < 0.01, ***P < 0.001.
Intracellular dye fills
MN5 intracellular dye fill was essentially carried out as described previously (Duch et al., 2008; Kuehn & Duch, 2013; Vonhoff et al., 2013). Briefly, MN5 was cleaned with protease as for patch-clamping (see above; Ryglewski & Duch, 2012), and then rinsed with normal saline using two Pasteur pipettes. Filamented borosilicate glass capillaries (Sutter Instruments; o.d. 1.0 mm, i.d. 0.5 mm) were pulled with a Sutter Flaming/Brown P97 microelectrode puller to get a tip resistance of approximately 60 MΩ when filled with 2 M potassium acetate (KAc) with neurobiotin (Vector Laboratories) and tetramethylrhodamine-dextran 3000 (TRITC-dextran 3000; Invitrogen). Glass microelectrode tips were filled through capillary action by putting the electrode upside down into the dye solution with ~7% neurobiotin and TRITC-dextran 3000, each in 2 M KAc. The shaft was filled with 2 M KAc. To avoid dilution of the tip solution, an air bubble was left between the tip and shaft.
After cleaning, MN5 somata as well as the electrode tip were readily visible in bright light using a 40 ×/1.0 DIC VIS-IR water dipping lens. After MN5 soma impalement, the neuron was filled iontophoretically with the dextran/neurobiotin mixture using a positive current of ~0.5 nA (up to 1 nA) amplitude for 5–10 min. After removal of the electrode, the preparations were fixed immediately for 40 min in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) followed by several quick washes in 0.1 M PBS. Then, preparations were washed for 6 × 10 min with PBS Triton-X 0.5%. This was followed by overnight incubation with 1 : 500 streptavidin coupled to Cy3 (Invitrogen) in 0.1 M PBS to detect the neurobiotin label. After incubation, preparations were rinsed with 0.1 M PBS followed by an ascending ethanol series (50, 70, 90, 100%) for 10 min each. Preparations were cleared and mounted in methylsalicylate (Sigma, Germany) on glass slides, covered with 100-μm-thick glass coverslips and sealed with clear nail polish. After drying, label was visualized using a Leica SP8 confocal laser-scanning microscope (Leica, Germany).
Immunohistochemistry
The animals used in this study were expressing mCD8-GFP in MN5, which could be visualized without enhancement with antibodies. Sodium channel immunohistochemistry was essentially carried out as published (Kuehn & Duch, 2013) with small changes. Briefly, preparations were dissected, fixed for 50 min in 4% PFA and then washed overnight in 0.1 M PBS. The next day, preparations were washed for 6 × 30 min in PBS Triton-X 0.5% and then blocked with 0.2% bovine serum albumin (BSA) in PBS Triton-X 0.3% for 2 h. This was followed by primary antibody incubation for 2 nights at 4 °C in 0.1 M PBS with 0.2% BSA and 0.3% Triton-X on a shaker. For sodium channel immunolabel, Millipore rabbit polyclonal anti-sodium channel, voltage-gated, Pan (SP19 Segment) was used at a concentration of 1 : 400. Specificity for Drosophila VGSC was confirmed previously (Kuehn & Duch, 2013).
Preparations were then rinsed with 0.1 M PBS for 6 × 30 min. For detection of sodium channel immunolabel, donkey anti-rabbit secondary antibody coupled to Cy5 (Jackson Immunoresearch) was used at a concentration of 1 : 400 in 0.1 M PBS overnight at 4 °C on a shaker. After secondary antibody incubation the preparations were rinsed for 6 × 30 min with PBS followed by an ascending ethanol series (50, 70, 90, 100%), and then mounted in methylsalicylate on 188-μm-thick metal slides with a ~0.7-cm-wide circular hole with coverslips glued to it using super glue. Preparations were covered with 100-μm-thick coverslips and sealed with nail polish. After drying, immunolabel was visualized using a Leica SP8 confocal laser-scanning microscope (Leica, Germany).
Image acquisition and quantitative morphometry
For intracellular dye fills, images were acquired with a Leica SP8 confocal laser-scanning microscope using a 40 ×/1.30 oil immersion objective. For VGSC immunolabel, a 20 ×/0.75 IMM CORR CS2 lens was used in combination with oil. Image resolution was 1024 × 1024 pixels with z-steps of 0.34 μm for VGSC immunolabel, and 0.29 μm for intracellular dye fills. Cy3 was excited with a krypton laser at 568 nm, and detected between 575 and 620 nm with a photomultiplier. Cy5 was excited with a helium neon laser at 633 nm, and detected with a super-sensitive Galliumarsenidphosphid Hybrid-Detector (HyD) between 640 and 700 nm. Very low laser intensities between 0.1 and 0.5% were required when used in combination with the HyD. Residual mCD-8-GFP expressed in MN5 (see above) was visualized using an argon laser with excitation wavelengths at 488 nm, and detected between 500 and 530 nm with a photomultiplier.
Images were further processed with Amira 5.3.3 (Mercury Systems). Geometric reconstructions were conducted with custom Amira (AMIRA 4.1.1 software, TGS) plug-ins as previously described (Schmitt et al., 2004; Evers et al., 2005). Data were exported to Microsoft Excel and SPSS 20 (IBM; New York, USA) for further analysis. For figure production, images were exported as tiff files, and further assembled and labeled in figure panels with CorelDrawX6 (Corel).
Statistical analysis
Error bars signify standard deviation (SD), if not otherwise specified. In some cases standard error (SEM) was chosen for reasons of figure clarity. Statistical differences were tested with one-way ANOVA. In case of significant F-values, pair-wise post hoc comparisons were conducted with Fisher’s least significant difference (LSD) test. Pair-wise post hoc comparisons were conducted between distinct developmental stages, so that type 1 error inflation was assumed minimal and no α-correction was conducted. All statistical analyses were conducted with SPSS 20 (IBM; New York, USA). P-values are signified as follows: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Results
The adult MN5 is a monopolar motoneuron with its soma located in the mesothoracic neuropil, a dendritic tree of more than 6500 μm total length (Vonhoff & Duch, 2010), and axon terminals on the two dorsal-most fibers of the DLM. During flight motor behavior MN5 fires tonically at approximately 5–20 Hz (Gordon & Dickinson, 2006). As mentioned above, MN5 is born embryonically, but remains developmentally arrested through the larval stages, until it starts growing its axon and dendrites during early pupal life (Consoulas et al., 2002; Vonhoff et al., 2013).
Action potentials can first be evoked at pupal stage P5 and coincided with the onset of dendrite growth
The occurrence of firing responses in MN5 coincided with the onset of dendritic growth. At pupal stage P5 early (P5e; ~12.5 h after puparium formation; APF), the primary neurite of MN5 was bare of dendrites (Fig. 1A; Vonhoff et al., 2013) and no action potentials could be evoked by somatic current injection (Fig. 1C). Similarly, no action potentials could be evoked at earlier pupal stages, such as P4 (not shown). By contrast, at pupal stage P5 late (P5 l), the primary neurite gave rise to numerous dendritic branches (Fig. 1B), and somatic current injections resulted in firing responses of MN5 (Fig. 1D). The onset of firing at pupal stage P5 coincided with other developmental hallmarks during metamorphosis, such as the onset of flight muscle growth (Costello & Wyman, 1986), expression of nicotinic acetylcholine receptors (nAChRs; Vonhoff et al., 2013) and the onset of dendrite growth in MN5 (Consoulas et al., 2002; Vonhoff et al., 2013; Fig. 1E).
Fig. 1.
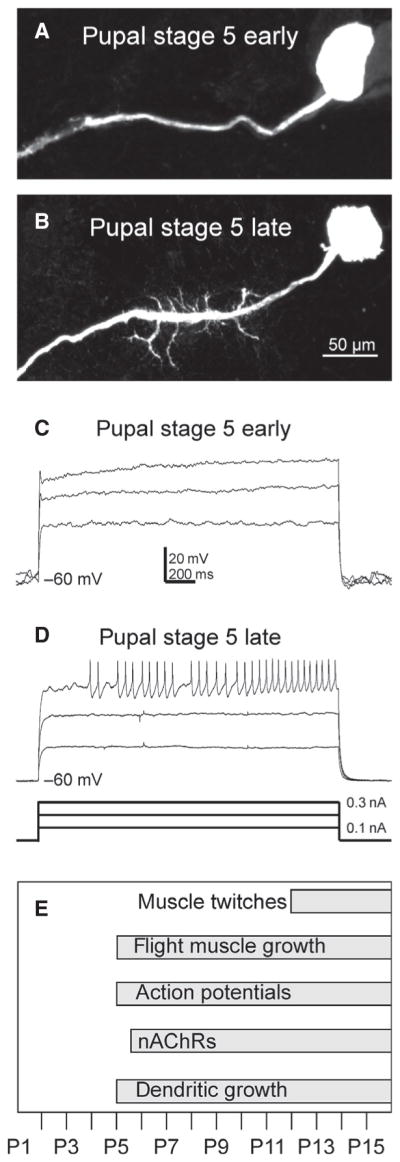
MN5 dendrite growth and evoked firing start at P5. Pupal stage P5 includes the sub-stages P5 early [~12.5 h after puparium formation (APF)] and P5 late (~16 h APF). In P5 early, MN5 had not yet developed any dendrites (A), whereas in P5 late some dendrites were clearly visible (B). Different amplitude (0.1–0.3 nA) depolarizing somatic current injections did not elicit any firing response in P5 early (C). In P5 late, tonic firing after a delay to the first spike was reliably evoked (D). The appearance of first action potentials coincided with other hallmarks during metamorphosis (E).
During pupal life action potentials progressively become larger and sharper, but intermittently show a calcium component
The onset of dendrite growth at P5 is followed by rapid growth and branching until P10–P12 (Fig. 2A, P7, P8, P12, top three images). Between P12 and P15 (pharate adult; Fig. 2A, P15, bottom image), growth rate is slower and dendrites are refined (Vonhoff et al., 2013). MN5 firing responses changed along with dendritic differentiation. During the early stages of dendrite growth, action potentials were small and broad (Fig. 2B, top trace, P5), and then developed a plateau-like shoulder at P7 (Fig. 2B, second trace from top, see arrow) that was most pronounced at P8 (Fig. 2B, third trace from top, see arrow, and Fig. 2C, left black trace) but still detectable at P10 (Fig. 2B, fourth trace from the top, see arrow). Between P7 and P10, this shoulder, as well as action potential amplitude, was calcium dependent as revealed by application of the calcium channel blocker cadmium (500 μM; see exemplified for P8, Fig. 2C, left light-gray trace). As development proceeded this calcium-dependent component disappeared and could not be detected anymore at P12 (Fig. 2C), the first pupal stage when action potentials appeared adult-like (Fig. 2B, third trace from bottom, and Fig. 2C, right black trace) and muscle twitches can first be observed through the pupal case (Fig. 1E).
Quantification of developmental changes of resting membrane potential, firing threshold, action potential amplitude, half-maximum amplitude action potential widths and time to peak (Fig. 2D–F; for values, see Table 1) revealed that already at P5 (−54.7 ± 8.8 mV) MN5 had a negative membrane potential that did not change significantly until P15 (−55.2 ± 2.0 mV; Fig. 2E, black bars). By contrast, in the adult MN5 the resting membrane potential was significantly more negative (−67.8 ± 5.5 mV; Fig. 2E, black bars) as compared with any pupal stage. At P5, the firing threshold was significantly more positive as compared with any pupal stages older than P7 (Fig. 2E, gray bars; see also Table 1). The firing threshold was not altered significantly at any stage between P8 and adult (Fig. 2E, gray bars; see also Table 1).
Action potential amplitudes progressively increased as metamorphosis proceeded (Fig. 2F, black bars; for values, see Table 1). Action potential amplitudes at P5 were below 10 mV on average, whereas in the adult action potential amplitudes were ~45 mV on average (Table 1). Although there was a continuous increase in action potential amplitude between P5 and P12 (Fig. 2F, black bars), pair-wise comparisons revealed a statistically significant increase of action potential amplitude between P8 and P10 (P < 0.05, ANOVA, LSD post hoc comparison). No significant changes in amplitude were detected between P10 and adulthood (Fig. 2F, black bars; P > 0.2, ANOVA, LSD post hoc comparisons). Along with an increase in action potential amplitude during pupal life, its width at half-maximum amplitude changed (Fig. 2F, gray bars) as did time to peak (Table 1). Action potential width increased significantly between P5 and P8 (Fig. 2F, gray bars; ANOVA, LSD post hoc comparisons, P < 0.001), to then decrease significantly between P8 and P10 (Fig. 2F, gray bars; ANOVA, LSD post hoc comparisons, P < 0.001), and remain unchanged between P12 and adulthood (Fig. 2F, gray bars; ANOVA, LSD post hoc comparisons, P > 0.2). In other words, while action potentials progressively increased in amplitude during pupal life, action potential width was transiently increased at P8 and then continuously decreased until adulthood (Fig. 2F, gray bars; Table 1).
In summary, the occurrence of firing responses in MN5 coincided with the onset of dendrite growth. At P5e the primary neurite of MN5 was bare of dendrites (Fig. 1A; Vonhoff et al., 2013) and no action potentials could be evoked (Fig. 1C), whereas at P5 l MN5 displayed some dendrites (Fig. 2B) and often responded with firing upon somatic current injection (Figs 1D and 2B, top trace). As MN5 dendrites grew rapidly until P10, action potentials were broad and contained a calcium-dependent component. During slower dendritic growth between P12 and adulthood, action potential amplitude, threshold, time to peak and width remained unaltered, but resting membrane potential was significantly decreased post-adult emergence.
Immunolabeling of VGSCs during pupal life
MN5 action potentials are mediated by VGSCs. Consistent with a lack of firing responses prior to P5 l, no immunolabel for VGSCs was detected in MN5 or in any other motoneuron soma in the mesothoracic neuromere at pupal stages P3 and P4 (data not shown). However, at early pupal stages some unidentified processes of projection neurons were immunopositive (Fig. 3A and B, white arrowheads), but these were not found at later pupal stages (Fig. 3C and D). From pupal stage P5 on, neuronal somata located in the ganglionic cortex of the mesothoracic neuromere showed weak (just above background) immunopositive label for VGSCs (see white asterisks in Fig. 3A and Ai). By contrast the neuropil regions (Fig. 3Ai, surrounded by dotted white line) were devoid of immunopositive label for VGSCs, and no immunopositive tracts or commissures were detected (Fig. 3A and Ai). This indicated that starting with pupal stage P5, the first expression of VGSCs could be detected in neuronal somata, but channels were not yet localized to axons in detectable concentrations.
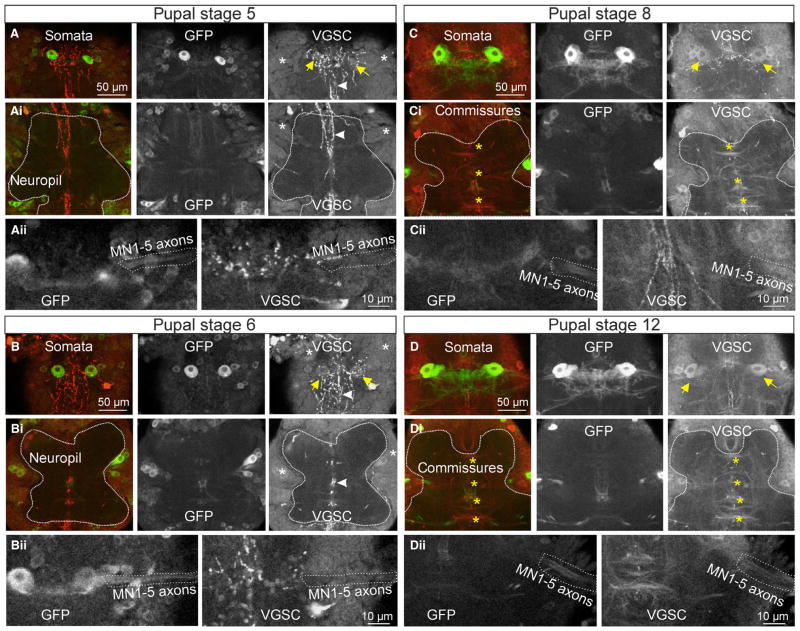
Fig. 3.
In the ventral nerve cord, immunopositive label for voltage-gated sodium channels (VGSCs) is first detectable in neuronal somata at P5 but not localized to axons before P8. MN5 was identified throughout pupal development by targeted expression of mCD-8-GFP (green, middle gray panels), and immunocytochemistry for VGSCs was conducted in the same preparations (red, right gray panels). Confocal images stacks were processed to depict representative projection views of 15 sections (2 μm each) through the dorsal somata layers (A–D), representative projection views of 15 sections through the thoracic motor neuropil (Ai, Bi, Ci, Di), and representative projection views of five sections through the axons of MN1–5 at the root of the motor nerve (Aii, Bii, Cii, Dii) for stages P5 (A–Aii), P6 (B–Bii), P8 (C–Cii) and P12 (D–Dii). (A) The ability of MN5 to fire action potentials at P5 coincided with the onset of VGSC expression in the somata of MN5 (right panel, yellow arrows) and other unidentified neurons (right panel, white asterisks) in the ventral nerve cord. In addition, the processes of some unidentified descending neurons were immunopositive for VGSCs (A, Ai, white arrowheads), but these disappeared at later stages. At P5, VGSC immunolabel did not reveal tracts or commissures in the ventral nerve cord neuropil (Ai, neuropil surrounded by dotted white line). MN1–5 axons were devoid of immunopositive label (see dotted lines in Aii). Immunolabel for VGSCs was qualitatively similar at pupal stage P6 (B–Bii), with positive label in neuronal somata (B, Bi, white asterisk) including MN5 (B, Bi, yellow arrows). As at P5, tracts and commissures in the neuropil (Bi, neuropil surrounded by dotted white line) as well as MN1–5 axons (Bii, dotted white line) were devoid of VGSC-immunopositive signal. At pupal stages P8 and P12, MN5 somata clearly expressed VGSCs (yellow arrows in C, D, right panels). At these stages, sodium channel immunostaining clearly revealed prominent commissures in the ventral nerve cord (see yellow asterisks in Ci, Di). Similarly, MN1–5 axons were immunopositive (see dotted lines in Cii, Dii, right panels). [Color version of figure available online].
Corresponding with that, the somata of MN5 on both sides of the mesothoracic neuromere showed the first VGSC-immunopositive label at pupal stage P5 (Fig. 3A, see yellow arrows), but no immunopositive label could be detected in MN1–5 axons (Fig. 3Aii, see dotted white line). At this stage, sodium-dependent action potentials could first be evoked by somatic current injections, though not yet reliably (in four out of 12 preparations; Fig. 1).
At pupal stage P6 (Fig. 3B–Bii), the immunopositive signal intensity for VGSCs through neuronal somata in the cortex of the mesothoracic neuromere (Fig. 3B and Bi, see white asterisks) was increased compared with P5 (Fig. 3A and Ai, see white asterisks). However, no immunopositive signal for VGSCs could be detected in the mesothoracic motor neuropil (Fig. 3B and Bi, surrounded by dotted white line), or in tracts or commissures. Similarly, in MN5, expression of VGSCs was detected in the soma (Fig. 3B, yellow arrows), but the axons of MN1–5 were devoid of immunopositive label (Fig. 3Bii, see dotted white line). At this stage, sodium-dependent action potentials could reliably be evoked in MN5 by somatic current injection (Figs 1 and 2).
At pupal stage P8, neuronal somata in the ganglionic cortex as well as MN5 somata (Fig. 3C, yellow arrows) were immunopositive for VGSCs. However, in contrast to earlier stages, at P8, tracts and commissures were immunopositive for VGSCs (Fig. 3Ci, yellow asterisks), indicating that sodium channels became localized to axons at this stage of pupal development. Correspondingly, at P8, immunopositive signal for VGSCs could first be detected in the axons of MN1–5 (Fig. 3Cii, dotted white line). At this stage, somatic current injection reliably evoked action potentials in MN5, but these were broader and of lower amplitude compared with mature spikes (Fig. 2).
At pupal stage P12, both neuronal somata in the ganglionic cortex and MN5 somata on both sides of the mesothoracic neuromere (Fig. 3D, yellow arrows) still showed VGSC-immunopositive signal. However, localization to axons became more prominent compared with pupal stage P8 (Fig. 3C), as axons within commissures (Fig. 3Di, yellow asterisks) as well as MN1–5 axons (Fig. 3Dii) were clearly immunopositive for VGSCs. Similarly, in the adult axon tracts, commissures and MN1–5 axons were prominently immunopositive for VGSCs (Kuehn & Duch, 2013). However, in the adult, MN5 soma and primary neurite were devoid of immunopositive label, whereas during pupal stages neuronal somata, including MN5, showed immunopositive signal (Fig. 3).
In summary, the first sodium channel expression in neuronal somata was observed at pupal stages P5 and P6, but protein levels in axon tracts, commissures and MN1–5 axons were below detection threshold. However, sodium-dependent action potentials could first be evoked by somatic current injections at these stages. Localization of VGSCs to axons was first observed by immunolabeling at pupal stage P8 and became more pronounced at pupal stage P12, when action potentials reached adult-like amplitudes (Fig. 2). Quantification of immunolabel intensities was not attempted because antibody penetration, histology and laser penetration differed between different pupal stages. However, this did not affect our qualitative results of the onset of detectable expression at P5 (Fig. 3A), and progressive localization to axons starting at P8 (Fig. 3C) and continuing via P12 (Fig. 3D) till adulthood (Kuehn & Duch, 2013).
Calcium channels were the first voltage-gated channels expressed in MN5 during metamorphosis
To relate the observed developmental changes in excitability and action potential shape to ionic currents in the membrane of MN5, we next studied VGCCs and voltage-gated potassium currents through pupal life by whole-cell voltage-clamp recordings.
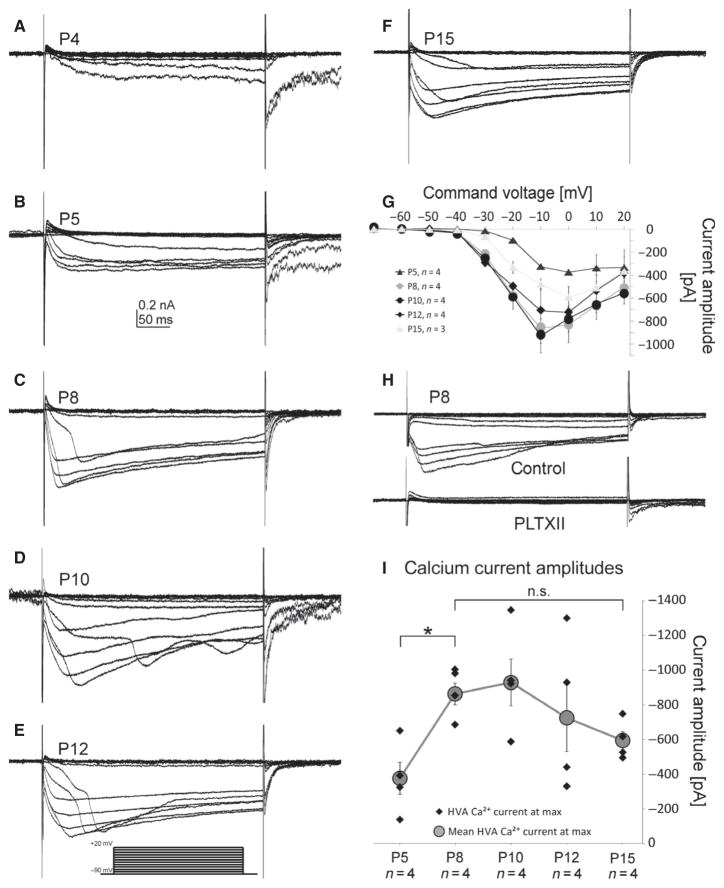
Somatic voltage-clamp recordings from MN5 revealed HVA calcium currents at pupal stage P4 (Fig. 4A), which was clearly prior to the onset of dendrite growth (Fig. 1; Vonhoff et al., 2013), as well as before sodium channel expression could be detected by immunocytochemistry (Fig. 3) and firing responses could be evoked by somatic current injection (Figs 1 and 2). Calcium currents were elicited by 200-ms depolarizing voltage steps from −90 mV to +20 mV in 10-mV increments from a holding potential of −90 mV. Recordings were conducted in the presence of VGSC as well as potassium channel blockers to isolate calcium currents (see Materials and methods; Ryglewski et al., 2012). At all stages (P4–P15; Fig. 4A–F), pupal HVA calcium current activated slower compared with HVA and low-voltage-activated (LVA) calcium currents in the adult MN5 (Ryglewski et al., 2012). Quantification of onset kinetics as well as tail currents was not attempted, because space-clamp conditions may vary during different stages of rapid dendritic growth (Fig. 4A–F). At all stages when dendrite growth was well underway (P8 and later), the appearance of staircase-like calcium currents (Fig. 4C–F) was likely due to non-uniform depolarization of the membrane and hints at the calcium channels being localized to dendritic regions (Carlin et al., 2009). Activation voltage was between −40 and −30 mV (Fig. 4G) through all pupal stages, the maximum amplitude was reached at 0 mV (P5, P12 and P15) or −10 mV (P8, P10; Fig. 4G). At all pupal stages, all HVA calcium current was blocked by bath application of the cacophony-specific spider toxin PLTXII (Fig. 4H; exemplified for P8). The specificity of PLTXII for cacophony calcium channels has previously been shown at the neuromuscular junction (Branton et al., 1987) and in the adult flight motoneuron MN5 (Ryglewski et al., 2012). The maximum calcium current amplitude increased between P5 and P10 to then decrease again between P10 and P15 (Fig. 4G). However, statistical analysis of developmental changes in maximum calcium current amplitudes revealed a significant increase between P5 and P8 (Fig. 4I; ANOVA, LSD post hoc comparison, P = 0.025). Through the remainder of pupal life between P8 and P15, the mean calcium current amplitude decreased from −880 ± 126 to −596 ± 97 pA, but this change was not statistically significant (ANOVA, LSD post hoc comparison, P = 0.248). In summary, calcium currents were present prior to voltage-gated sodium and potassium currents, and significant increases in current amplitude occurred only until P8, when the action potentials showed a pronounced calcium-dependent component. By contrast, potassium channel amplitudes increased later during development (see below).
Fig. 4.
HVA calcium current comes first during pupal life. MN5 calcium current as elicited by 200-ms depolarizing voltage steps from −90 to +20 mV in 10-mV increments from a holding potential of −90 mV. Leak was subtracted offline. HVA calcium currents were detectable from P4 on (A), and could reliably be evoked from P5 on through pupal life (B–F). With increasing complexity of the MN5 dendritic tree, the staircase-like appearance of HVA calcium current occurred (C–F). (G) IV-plot for HVA calcium currents at pupal stages P5, P8, P10, P12 and P15. At all stages HVA calcium current activated between −40 and −30 mV, with maximum amplitudes at 0 mV (P5, P12, P15) or at −10 mV (P8, P10), respectively (G). HVA calcium current was completely blocked by application of the cacophony calcium channel blocker PLTXII at a 20 nM concentration (H). The mean calcium current amplitude increased significantly between P5 and P8 (I, gray circles, P < 0.05, ANOVA with LSD pair-wise post hoc comparisons, for single values see black diamonds). Errors represent SEM Statistically significant difference was assumed at *P < 0.05.
Transient potassium current was present after calcium but before sustained potassium current
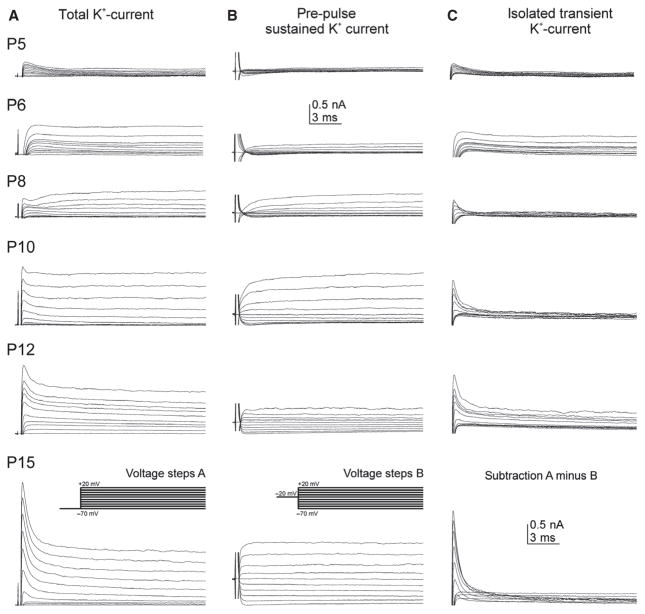
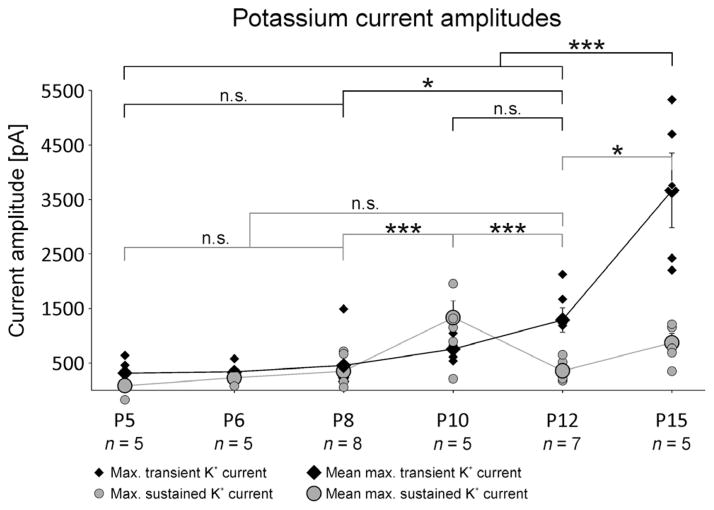
Voltage-gated potassium currents were recorded using a depolarizing voltage protocol with 400-ms pulses from −70 to +20 mV in 10-mV increments from two different holding potentials. Total voltage-gated potassium current, including transient as well as sustained currents, was evoked from a holding potential of −70 mV (Fig. 5A, see also inset in A). Potassium outward currents were first detected at pupal stage P5, and progressively increased in amplitude during pupal development to P15 (Fig. 5A). Potassium outward currents consisted of at least two different components, a transient A-type-like current and a sustained one. By using a 200-ms pre-pulse to −20 mV the transient potassium current was inactivated, revealing a sustained voltage-gated potassium current with an activation voltage between about −40 and −30 mV (Fig. 5B, see also inset in B). For better visibility, capacitance artifacts at pulse onset were clipped (Fig. 5B). The first stage when sustained potassium outward current could reliably be detected was pupal stage P6 (Fig. 5B, second trace from top). It increased in amplitude between P6 and P10 (Fig. 5B, second–fourth trace) to then decrease at P12 and increase again toward pharate adult (Fig. 5B, traces 3–6). Figure 6 shows quantification and statistical analysis of developmental changes in sustained potassium current amplitudes. The transient voltage-gated potassium current was isolated by offline subtraction of sustained current (Fig. 5B) from total current (Fig. 5A), and is depicted for each stage in Fig. 5C. Transient potassium current activated between −60 and −50 mV was first reliably detectable at pupal stage P5 (Fig. 5A and C, top traces), and progressively increased in amplitude during pupal life (Fig. 5C, for quantification see Fig. 6, black diamonds).
Fig. 5.
Voltage-gated potassium currents are present in MN5 from P5 on. The total voltage-gated potassium current was evoked by 400-ms depolarizing voltage steps from −70 to +20 mV from a holding potential of −70 mV (A, also see inset in bottom trace). Sustained voltage-gated potassium current was elicited from a holding potential of −70 mV with a 200-ms pre-pulse to −20 mV before voltage steps from −70 mV to +20 mV in 10-mV increments were applied (B, also see inset in bottom trace). The pre-pulse inactivated transient voltage-gated potassium currents. Transient voltage-gated potassium current was isolated (C) by offline subtraction of sustained currents (B) from total potassium currents (A). Transient voltage-gated potassium currents were detectable from P5 on (A, C, top traces), with progressively increasing amplitudes and sharpness through pupal development (A, C) until P15 (bottom trace). Transient currents activated between −50 and −60 mV. The current peaked within 3 ms (C). Sustained potassium current activated slower. At all stages, the activation voltage was between −30 and −40 mV (B). Sustained current amplitude increased until P10 (B, third trace from the bottom). After that, current amplitude was decreased at P12 and increased again at P15 (B, bottom two traces).
Fig. 6.
MN5 potassium current amplitudes during metamorphosis. Maximum amplitudes of transient (black diamonds) and sustained potassium currents (gray circles) at pupal stages P5, P6, P6, P10, P12 and P15. Small symbols represent individual cells, large symbols represent mean values. Transient potassium current amplitude was determined at peak, whereas sustained potassium current amplitude was determined by the mean amplitude between 260 and 300 ms after pulse onset. Errors represent SEM. Data were analysed with ANOVA and LSD pair-wise post hoc comparisons. Statistically significant differences were assumed at *P < 0.05, ***P < 0.001.
Statistical analysis revealed that both transient and sustained potassium currents increased significantly in amplitude during metamorphosis, but transient current increased roughly by a factor of 11 between P5 and P15, whereas sustained current amplitude increased only four times between P6 and P15 (Fig. 6). Neither transient (Fig. 6, black diamonds) nor sustained potassium currents (Fig. 6, gray circles) showed statistically significant increases in their amplitudes between the first appearance and P8 (P = 0.716 and P = 0.507, respectively). Between P8 and P15, developmental increases in current amplitudes followed different time courses. Sustained potassium current amplitude increased transiently with a significant increase between P8 and P10 (P < 0.001), followed by a significant decrease between P10 and P12 (P < 0.001). It then increased again significantly between P12 and P15 (P = 0.018). In fact, the transient amplitude peak at P10 was not different from sustained current amplitude at P15 (P = 0.13; Fig. 6, gray circles). This transient peak of sustained potassium current at P10 coincided with the gradual disappearance of the calcium-dependent shoulder of the action potential, which was most pronounced at P8 (Fig. 2B).
By contrast, transient potassium current amplitude increased continuously between P5 and P15, with statistically significant increases between P8 and P12 (from 435 ± 142 to 1290 ± 226 pA, P = 0.026), and a highly significant increase between P12 and P15 (from 1290 ± 226 to 3663 ± 685 pA; Fig. 6, black diamonds, P < 0.001). Therefore, transient potassium current amplitude increased by about 1000 pA between P5 and P12 to then increase by another 2300 pA between P12 and P15, thus resembling an exponential increase during metamorphosis (Fig. 6). In contrast, sustained potassium current amplitude peaked at P10 (Fig. 6) when it was exceptionally large at 1160 ± 625 pA (Figs 5, third trace from bottom, and 6).
Cacophony currents are required for normal dendritic growth during pupal life
To address a potential role of activity-dependent calcium influx through somatodendritic voltage-gated cacophony channels, we expressed UAS-cacophony-RNAi (cac-RNAi) together with UAS-dicer2 (UAS-dcr2; Dietzl et al., 2007) in MN5 under the control of two different GAL4 drivers, namely D42 and C380, to then quantify dendritic architecture in the adult by intracellular dye fills, confocal microcopy and subsequent quantitative three-dimensional dendritic architecture reconstructions (Evers et al., 2005, 2006). D42-GAL4 and C380-GAL4 express in flight MN1–5, multiple other motoneurons, and in about 30 unidentified neurons per hemisegment (Boerner & Duch, 2010). To prevent expression in cholinergic interneurons and cholinergic sensory neurons, the Cha-GAL80 transgene was included. C380-GAL4 expression in MN5 starts at pupal stage P5, whereas D42-GAL4 expression in MN5 starts at pupal stage P6, and both express through adulthood from then on (Vonhoff et al., 2013). Therefore, expression of the cac-RNAi transgene covers all pupal stages when action potentials could reliably be evoked by somatic current injection (Fig. 2). We have previously demonstrated that inclusion of UAS-drc2 effectively knocks down all somatodendritic cacophony-based calcium current in MN5 (Ryglewski et al., 2012). As compared with controls (Fig. 7A), knockdown of cacophony channels under the control of either C380-GAL4;; Cha-GAL80 (Fig. 7B) or D42-GAL4, Cha-GAL80 (Fig. 7C) did not affect the characteristic overall shape of adult MN5 dendrites, but it resulted in an obvious reduction in the number of dendritic branches. Quantification demonstrated a significant reduction in total dendritic length (Fig. 7D; P < 0.01) and in the total number of dendritic branches (Fig. 7E; P < 0.01) in both knockdowns. The number of branches was reduced through all branch orders (Fig. 7F), indicating that calcium influx through cacophony channels was required for normal dendritic growth and branching through all pupal stages of postembryonic dendritic growth (see Discussion).
Fig. 7.
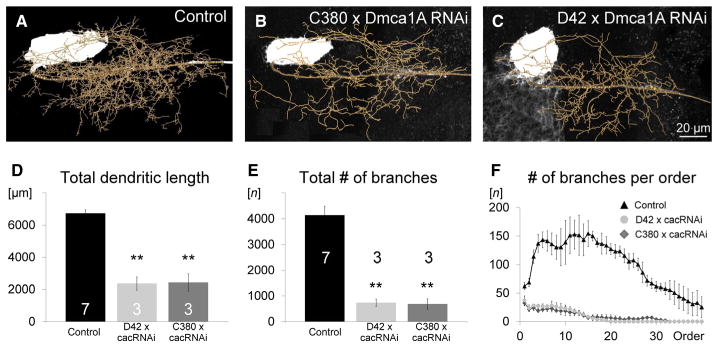
Cacophony-based HVA calcium channels are required for MN5 dendritic growth during normal development. Representative geometric dendritic reconstructions superimposed on projection views of adult MN5 intracellular fills in a control (A) and following cac-RNAi knockdown in MN5 under the control of C380-GAL4;; Cha-GAL80 (B) and D42-GAL4, Cha-GAL80 (C). Total dendritic length (D) and the number of dendritic branches (E) were significantly reduced by more than 60% in both knockdown genotypes (ANOVA with LSD pair-wise post hoc comparisons, **P < 0.01). Following expression of cac-RNAi in MN5 under the control of either D42-GAL4, Cha-GAL80 (gray circles) or C380-GAL4;; Cha-GAL80 (gray diamonds), the number of branches was reduced through all branch orders as compared with control (F, black triangles).
Discussion
Structural and physiological differentiation start simultaneously at early pupal life
This study investigated the development of voltage-gated membrane currents, excitability and action potential shape in relation to the time course of dendritic growth of the identified Drosophila flight motoneuron, MN5 (Fig. 8). As mentioned above, although MN5 is born embryonically, it remains developmentally arrested until early pupal life. Both first action potentials as well as first dendritic branches start forming at stage P5, 12.5–16 h APF. This coincides with the expression of VGSCs and A-type potassium currents. Only about 8–12 h later, at stage P6 (25 h APF), action potentials can reliably be evoked and rapid dendritic growth is well underway (Fig. 8). At the same time, MN5 also starts expressing nicotinic acetylcholine receptors (Vonhoff et al., 2013; Fig. 1E), the anlagen of the developing target muscle have already formed (Fernandes et al., 1991) and started growing (Costello & Wyman, 1986), and the MN5 axon is growing over the developing muscle anlagen (Consoulas et al., 2002). Therefore, in the developmentally arrested MN5, multiple different aspects of structural and physiological differentiation start within a narrow time window at early pupal life, i.e. stage P5 (Figs 1E and 8). This is consistent with global hormone signals that are known to govern multiple aspects of CNS development and neuromuscular differentiation during insect metamorphosis (Consoulas et al., 2000). In fact, the insect molting steroid hormone, 20-hydroxyecdysone (20HE), is elevated during puparium formation and has been demonstrated to mediate mushroom body neuron neurite (Matheson & Levine, 1999) and motoneuron dendrite growth (Kraft et al., 1998), myoblast proliferation (Luedeman & Levine, 1996), as well as expression of VGCCs but not potassium channels in motoneurons (Grünewald & Levine, 1998). Thoracic motoneurons, such as MN5, express ecdysone receptor A, which has been associated with maturational events such as sprouting and synaptogenesis (Talbot et al., 1993; Truman, 1996). Therefore, it seems plausible that many aspects of MN5 postembryonic differentiation may be mediated by 20HE.
Fig. 8.
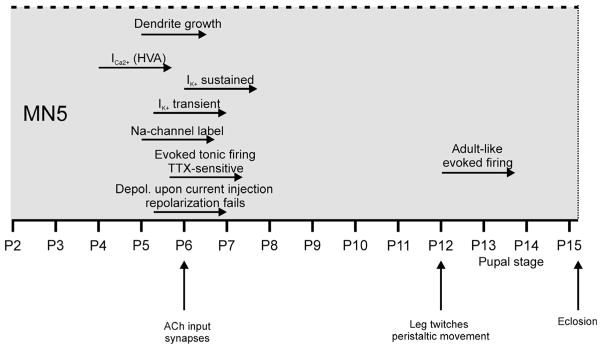
Summary of developmental events observed in MN5. Origins of horizontal arrows represent developmental onset of respective events.
However, most strikingly, HVA calcium channels are already expressed at pupal stage P4, about 5 h before any other sign of structural or physiological differentiation is detectable in MN5. In addition, in MN5 action potential shape is transiently modified by a calcium-dependent component, and activity-dependent mechanisms have previously been demonstrated to affect motoneuron dendrite growth in addition to steroid signals (Duch & Mentel, 2004; Vonhoff et al., 2013). Below we will first discuss the relation between sequential ion channel development and MN5 spike shape, and then discuss the role of activity and HVA calcium currents in motoneuron dendrite growth.
Stereotyped developmental changes in spike shape correspond to the sequential acquisition of VGCCs, VGSCs and voltage-gated potassium channels
Given the importance of intrinsic electrical properties for a properly functioning nervous system, surprisingly little data are available on the development of ionic currents in neurons. For example, some work has been published on electrical properties of neurons during metamorphosis of the sphinx moth, Manduca sexta, and on vertebrate embryonic development of spinal neurons (O’Dowd et al., 1988; McCobb et al., 1989, 1990), or also Purkinje cells (Yool et al., 1988). One striking feature in Manduca and Xenopus is the combination of calcium conductances with low expression levels of potassium channels during specific stages of development. This results in the generation of calcium-dependent action potentials, which can also cause plateau potentials (Spitzer & Lamborghini, 1976; Holliday & Spitzer, 1990; Lockery & Spitzer, 1992; Gu & Spitzer, 1995; Duch & Levine, 2000, 2002; Mercer & Hildebrand, 2002; Mercer et al., 2004). By contrast, calcium-dependent action potentials were not observed in Drosophila embryonic development (Baines & Bate, 1998). Similar to Manduca and Xenopus, during Drosophila pupal development we found that calcium currents were the first to be functionally expressed (Fig. 8). Moreover, HVA calcium current amplitudes reached near maximum amplitudes at pupal stage P8 when outward potassium currents were still expressed at low levels. As a result, between P7 and P10 both action potential amplitude and width showed a calcium-dependent component, which was most pronounced at P8. After P10, this calcium-dependent component was not detectable anymore, although HVA current amplitude did not change significantly between P10 and pharate adult. However, the transient increase in sustained potassium current at P10, as well as the significant upregulation of A-type potassium current between P10 and P15 likely masked this calcium-dependent component. In addition, immunocytochemistry for VGSCs indicated a constant increase in channel expression between P6 and P12, when the sodium-dependent action potential has reached maximum adult-like amplitudes. Similarly, in multiple systems a developmental increase in neuronal sodium current density causes increasing dependency of action potentials on sodium as well as increasing action potential amplitudes. Simultaneously, developmental increases in the amplitudes of transient potassium currents narrow spike shape (O’Dowd et al., 1988; McCobb et al., 1990; Mori-Okamoto et al., 1993; Mercer & Hildebrand, 2002).
In summary, comparison of Drosophila embryonic (Baines & Bate, 1998) and postembryonic development (this study) with Xenopus central neuron (Spitzer & Ribera, 1998) and chick embryonic motoneuron development (McCobb et al., 1989, 1990) shows that there is no uniform order of channel appearance during electrophysiological differentiation of neurons. However, common themes are that immature action potentials are typically broader and may contain calcium components, or allow for calcium influx through voltage-dependent calcium channels, and that developmental increases in sodium and potassium current amplitudes sharpen action potentials during maturation. The specific types of potassium channels or calcium channels that are developmentally regulated differ markedly between different species and cell types within a species. For example, in MN5 the first calcium currents present are HVA currents, whereas the first calcium currents in hippocampal neurons (Yaari et al., 1987), dorsal horn precursor cells (Gottmann et al., 1988) and chick ciliary ganglia neurons (McCobb et al., 1989) are LVA currents. In fact, in most terrestrial vertebrates the first calcium current in spinal motoneurons during embryonic development seems to be a T-type LVA calcium current (for review, see Perrier & Hounsgaard, 2000). In MN5, pupal HVA calcium currents are mediated by cacophony at all stages tested (P4–P15). However, MN5 pupal cacophony-based HVA current differs qualitatively from adult HVA and LVA calcium current in MN5, both of which are also mediated by cacophony (Ryglewski et al., 2012). Therefore, cacophony channels can mediate at least three qualitatively different somatodendritic calcium currents as recorded from the soma of MN5.
In Xenopus embryonic development, different voltage-dependent potassium channels function to shape the developing action potential, depending on the type of neuron (Spitzer & Ribera, 1998). Recent studies demonstrate that transcription factors that determine neural identity may regulate which types of potassium channels are expressed in which types of central neurons (Wolfram et al., 2012).
Somatodendritic cacophony channels are required for MN5 normal dendrite growth
It is well known from multiple systems that activity-dependent calcium influx may act together with genetic programs as a key regulator of neuronal growth (Van Aelst & Cline, 2004; Lohmann & Wong, 2005), synaptogenesis (Niell et al., 2004), ion channel expression (Baines & Pym, 2006), and even neurotransmitter selection (Spitzer, 2012). We found functional HVA calcium currents already at pupal stage P4 (~12 h APF), which is clearly before dendrite outgrowth (Fig. 8) and synaptogenesis in MN5 (Vonhoff et al., 2013). At this early stage the anlagen for the developing DLM have already formed (Fernandes et al., 1991), and the axon of MN5 is growing towards the developing muscle anlagen (Consoulas et al., 2002). In Xenopus spinal neurons, axonal growth cones exhibit transient calcium elevations that are likely initiated by leak through voltage-dependent calcium channels (Spitzer & Ribera, 1998). Manipulation of transient calcium elevation in Xenopus neurons has demonstrated that the rate of axonal growth is inverted proportional to the frequency of growth cone calcium waves (Gomez & Spitzer, 1999). However, at present we have no experimental evidence for a function of VGCCs in MN5 axonal growth.
By contrast, MN5 dendrite growth is affected by neuronal activity (Duch et al., 2008; Vonhoff et al., 2013). In MN5, the ability to fire action potentials and excitatory cholinergic input appear at pupal stage P5, when HVA calcium currents are already present (Fig. 8). Therefore, the very moment MN5 becomes active, be it through action potential firing, synaptic input or other routes of activity, calcium entry through VGCCs is possible. We have recently demonstrated that normal MN5 dendritic growth rates require activity-dependent activation of the transcriptional regulator AP-1 (heterodimer of c-fos and c-jun) via cholinergic receptor and CaMKII activation (Vonhoff et al., 2013). In this study, we now demonstrate that MN5 cacophony-based HVA calcium currents are also required for normal rates of dendritic growth, because RNAi knockdown of cacophony channels causes a significant reduction in the number of dendritic branches and total dendritic length without affecting dendritic territory boundaries or the characteristic shape of the MN5 dendrites. This phenotype is reminiscent of what we previously observed in mutants for nAChRs, knockdown of CaMKII or inhibition of AP-1-dependent transcription (Vonhoff et al., 2013). This indicates that both calcium influx through nAChRs (Vonhoff et al., 2013) or through VGCCs may contribute to CaMKII- and AP-1-dependent dendritic growth regulation. Alternatively, activity-dependent calcium influx may act locally at dendritic sites to stabilize newly formed dendrites or synapses, as reported for retinal ganglion cells (Lohmann et al., 2002) or in zebrafish tectal neurons (Niell et al., 2004). The molecular toolkit available in Drosophila will now allow further dissection of the cell autonomous functional consequences of ionic current differentiation for structural development.
Acknowledgments
Support by the German Research Foundation to C.D. (Du 331/6-1) and by the US National Science Foundation (NSF; IOS-0949051) are gratefully acknowledged. The authors thank Corinna Herr for her help with fly keeping.
Abbreviations
- 20HE
20-hydroxyecdysone
- 4-AP
4-aminopyridine
- AP-1
heterodimer of c-fos and c-jun
- APF
after puparium formation
- BSA
bovine serum albumin
- DLM
dorsolongitudinal wing depressor muscle
- HVA
high-voltage-activated
- LSD
least significant difference
- LVA
low-voltage-activated
- MN5
motoneuron 5
- nAChR
nicotinic acetylcholine receptor
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PLTXII
Plectreurys tristis toxin II
- TEA-Br
tetraethylammoniumbromide
- TEA-Cl
tetraethylammonium-chloride
- TRITC-dextran 3000
tetramethylrhodamine-dextran 3000
- TTX
tetrodotoxin
- VGCC
voltage-gated calcium channel
- VGSC
voltage-gated sodium channel
References
- Bainbridge SP, Bownes MJ. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morph. 1981;6:57–80. [PubMed] [Google Scholar]
- Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Pym EC. Determinants of electrical properties in developing neurons. Semin Cell Dev Biol. 2006;17:12–19. doi: 10.1016/j.semcdb.2005.11.003. Review. [DOI] [PubMed] [Google Scholar]
- Boerner J, Duch C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol. 2010;518:2437–2455. doi: 10.1002/cne.22346. [DOI] [PubMed] [Google Scholar]
- Branton WD, Kolton L, Jan YN, Jan LY. Neurotoxins from Plectreurys spider venom are potent presynaptic blockers in Drosophila. J Neurosci. 1987;7:4195–4200. doi: 10.1523/JNEUROSCI.07-12-04195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin KP, Bui TV, Dai Y, Brownstone RM. Staircase currents in motoneurons: insight into the spatial arrangement of calcium channels in the dendritic tree. J Neurosci. 2009;29:5343–5353. doi: 10.1523/JNEUROSCI.5458-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoulas C, Duch C, Bayline RJ, Levine RB. Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull. 2000;53:571–583. doi: 10.1016/s0361-9230(00)00391-9. [DOI] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver PF, Truman JW. Metamorphosis of the cerebral neuroendocrine system in the moth Manduca sexta. J Comp Neurol. 1986;249:186–204. doi: 10.1002/cne.902490206. [DOI] [PubMed] [Google Scholar]
- Costello WJ, Wyman RJ. Development of an indirect flight muscle in a muscle-specific mutant of Drosophila melanogaster. Dev Biol. 1986;118:247–258. doi: 10.1016/0012-1606(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Duch C, Levine RB. Remodeling of membrane properties and dendritic architecture accompanies the postembryonic conversion of a slow into a fast motoneuron. J Neurosci. 2000;20:6950–6961. doi: 10.1523/JNEUROSCI.20-18-06950.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Levine RB. Changes in calcium signaling during postembryonic dendritic growth in Manduca sexta. J Neurophysiol. 2002;87:1415–1425. doi: 10.1152/jn.00524.2001. [DOI] [PubMed] [Google Scholar]
- Duch C, Mentel T. Activity affects dendritic shape and synapse elimination during steroid controlled dendritic retraction in Manduca sexta. J Neurosci. 2004;24:9826–9837. doi: 10.1523/JNEUROSCI.3189-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Bayline RJ, Levine RB. Postembryonic development of the dorsal longitudinal flight muscle and its innervation in Manduca sexta. J Comp Neurol. 2000;422:1–17. doi: 10.1002/(sici)1096-9861(20000619)422:1<1::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S. Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol. 2008;100:2525–2536. doi: 10.1152/jn.90758.2008. [DOI] [PubMed] [Google Scholar]
- Evers JF, Schmitt S, Sibila M, Duch C. Progress in functional neuroanatomy: precise automatic geometric reconstruction of neuronal morphology from confocal image stacks. J Neurophysiol. 2005;93:2331–2342. doi: 10.1152/jn.00761.2004. [DOI] [PubMed] [Google Scholar]
- Evers JF, Muench D, Duch C. Developmental relocation of pre-synaptic terminals along distinct types of dendritic filopodia. Dev Biol. 2006;297:214–227. doi: 10.1016/j.ydbio.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Fernandes JJ, Keshishian H. Nerve-muscle interactions during flight muscle development in Drosophila. Development. 1998;125:1769–1779. doi: 10.1242/dev.125.9.1769. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Bate M, Vijayraghavan K. Development of the indirect flight muscles of Drosophila. Development. 1991;113:67–77. doi: 10.1242/dev.113.1.67. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc Natl Acad Sci USA. 2006;103:4311–4315. doi: 10.1073/pnas.0510109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Dietzel ID, Lux HD, Huck S, Rohrer H. Development of inward currents in chick sensory and autonomic neuronal precursor cells in culture. J Neurosci. 1988;8:3722–3732. doi: 10.1523/JNEUROSCI.08-10-03722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald B, Levine RB. Ecdysteroid control of ionic current development in Manduca sexta motoneurons. J Neurobiol. 1998;37:211–223. doi: 10.1002/(sici)1097-4695(19981105)37:2<211::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Holliday J, Spitzer NC. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev Biol. 1990;141:13–23. doi: 10.1016/0012-1606(90)90098-4. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH. Morphological identification of the motor neurons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Comp Neurol. 1988;273:436–444. doi: 10.1002/cne.902730312. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Todman MG, Allen MJ, Davies JA, Bacon JP. Synaptogenesis in the giant-fibre system of Drosophila: interaction of the giant fibre and its major motorneuronal target. Development. 2000;127:5203–5212. doi: 10.1242/dev.127.23.5203. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Dennison J. The vocal motor neurons of Xenopus laevis: development of sex differences in axon number. J Neurobiol. 1990;21:869–882. doi: 10.1002/neu.480210605. [DOI] [PubMed] [Google Scholar]
- Kempermann G. New neurons for ‘survival of the fittest’. Nat Rev Neurosci. 2012;13:727–736. doi: 10.1038/nrn3319. Review. [DOI] [PubMed] [Google Scholar]
- Kraft R, Levine RB, Restifo LL. Steroid hormone enhancement of neurite outgrowth in identified insect motor neurons involves specific effects on growth cone form and function. J Neurosci. 1998;18:8886–8899. [PubMed] [Google Scholar]
- Kuehn C, Duch C. Putative excitatory and putative inhibitory inputs are localised in different dendritic domains in a Drosophila flight motoneuron. Eur J Neurosci. 2013;37:860–875. doi: 10.1111/ejn.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockery SR, Spitzer NC. Reconstruction of action potential development from whole-cell currents of differentiating spinal neurons. J Neurosci. 1992;12:2268–2287. doi: 10.1523/JNEUROSCI.12-06-02268.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Wong RO. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium. 2005;37:403–409. doi: 10.1016/j.ceca.2005.01.008. Review. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Luedeman R, Levine RB. Neurons and ecdysteroids promote the proliferation of myogenic cells cultured from the developing adult legs of Manduca sexta. Dev Biol. 1996;173:51–68. doi: 10.1006/dbio.1996.0006. [DOI] [PubMed] [Google Scholar]
- Matheson SF, Levine RB. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurobiol. 1999;38:27–45. doi: 10.1523/JNEUROSCI.18-21-08886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989;2:1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. The differentiation of excitability in embryonic chick limb motoneurons. J Neurosci. 1990;10:2874–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen IA. Sleeping neuroblasts. Curr Biol. 1993;3:904–906. doi: 10.1016/0960-9822(93)90233-e. [DOI] [PubMed] [Google Scholar]
- Mercer AR, Hildebrand JG. Developmental changes in the density of ionic currents in antennal-lobe neurons of the sphinx moth, Manduca sexta. J Neurophysiol. 2002;87:2664–2675. doi: 10.1152/jn.2002.87.6.2664. [DOI] [PubMed] [Google Scholar]
- Mercer AR, Kloppenburg P, Hildebrand JG. Plateau potentials in developing antennal-lobe neurons of the moth, Manduca sexta. J Neurophysiol. 2004;93:1949–1958. doi: 10.1152/jn.01050.2004. [DOI] [PubMed] [Google Scholar]
- Mori-Okamoto J, Okamoto K, Tatsuno J. Intracellular Mechanisms Underlying the Suppression of AMPA Responses by trans-ACPD in Cultured Chick Purkinje Neurons. Mol Cell Neurosci. 1993;4:375–386. doi: 10.1006/mcne.1993.1047. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. Review. [DOI] [PubMed] [Google Scholar]
- O’Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull. 2000;53:529–535. doi: 10.1016/s0361-9230(00)00386-5. Review. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Duch C. Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. J Neurophysiol. 2009;102:3673–3688. doi: 10.1152/jn.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Duch C. Preparation of Drosophila central neurons for in situ patch clamping. J Vis Exp. 2012;68 doi: 10.3791/4264. pii: 4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine RB, Duch C. Ca(v)2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J Physiol. 2012;590:809–825. doi: 10.1113/jphysiol.2011.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Evers JF, Duch C, Scholz M, Obermayer K. New methods for the computer-assisted 3-D reconstruction of neurons from confocal image stacks. NeuroImage. 2004;4:1283–1298. doi: 10.1016/j.neuroimage.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. Review. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13:94–106. doi: 10.1038/nrn3154. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Ribera AB. Development of electrical excitability in embryonic neurons: mechanisms and roles. J Neurobiol. 1998;37:190–197. Review. [PubMed] [Google Scholar]
- Spitzer NC, Kingston PA, Manning TJ, Conklin MW. Outside and in: development of neuronal excitability. Curr Opin Neurobiol. 2002;12:315–323. doi: 10.1016/s0959-4388(02)00330-6. Review. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Swyryd EA, Hogness DA. Drosophila tissues with different metamorphic responses to ecdysone express different receptor isoforms. Cell. 1993;73:1323–1337. doi: 10.1016/0092-8674(93)90359-x. [DOI] [PubMed] [Google Scholar]
- Truman JW. Steroid receptors and nervous system metamorphosis in insects. Dev Neurosci. 1996;18:87–101. doi: 10.1159/000111398. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Cline HT. Rho GTPases and activity-dependent dendrite development. Curr Opin Neurobiol. 2004;14:297–304. doi: 10.1016/j.conb.2004.05.012. Review. [DOI] [PubMed] [Google Scholar]
- Vonhoff F, Duch C. Tiling among stereotyped dendritic branches in an identified Drosophila motoneuron. J Comp Neurol. 2010;518:2169–2185. doi: 10.1002/cne.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonhoff F, Kuehn C, Blumenstock S, Sanyal S, Duch C. Temporal coherency between receptor expression, neural activity and AP-1-dependent transcription regulates Drosophila motoneuron dendrite development. Development. 2013;140:606–616. doi: 10.1242/dev.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram V, Southall TD, Brand AH, Baines RA. The LIM-homeodomain protein islet dictates motor neuron electrical properties by regulating K(+) channel expression. Neuron. 2012;75:663–674. doi: 10.1016/j.neuron.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaari Y, Hamon B, Lux HD. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987;235:680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]
- Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci USA. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ, Dionne VE, Gruol DL. Developmental changes in K+-selective channel activity during differentiation of the Purkinje neuron in culture. J Neurosci. 1988;8:1971–1980. doi: 10.1523/JNEUROSCI.08-06-01971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]