Abstract
Mitochondria are cellular power plants that supply ATP to power various biological activities essential for neuronal growth, survival, and function. Due to unique morphological features, neurons face exceptional challenges to maintain ATP and Ca2+ homeostasis. Neurons require specialized mechanisms distributing mitochondria to distal areas where energy and Ca2+ buffering are in high demand, such as synapses and axonal branches. These distal compartments also undergo development- and activity-dependent remodeling, thereby altering mitochondrial trafficking and distribution. Mitochondria move bi-directionally, pause briefly, and move again, frequently changing direction. In mature neurons, only one-third of axonal mitochondria are motile. Stationary mitochondria serve as local energy sources and buffer intracellular Ca2+. The balance between motile and stationary mitochondria responds quickly to changes in axonal and synaptic physiology. Furthermore, neurons are postmitotic cells surviving for the lifetime of the organism; thus, mitochondria need to be removed when they become aged or dysfunction. Mitochondria also alter their motility under stress conditions or when their integrity is impaired. Therefore, regulation of mitochondrial transport is essential to meet altered metabolic requirements and to remove aged and damaged mitochondria or replenish healthy ones to distal terminals. Defects in mitochondrial transport and altered distribution are implicated in the pathogenesis of several major neurological disorders. Thus, research into the mechanisms regulating mitochondrial motility is an important emerging frontier in neurobiology. This short review provides an updated overview on motor-adaptor machineries that drive and regulate mitochondrial transport and docking receptors that anchor axonal mitochondria in response to the changes in synaptic activity, metabolic requirement, and altered mitochondrial integrity. The review focuses on microtubule (MT)-based mitochondrial trafficking and anchoring. Additional insight from different perspectives can be found in other in-depth reviews.
Keywords: Mitochondrial transport, mitochondrial docking, mitophagy, motile mitochondria, stationary mitochondria, kinesin motors, dynein motors, syntaphilin, synaptic activity
Introduction
In the human brain, a resting cortical neuron consumes ~4.7 million ATP molecules per second to power various biological functions (Zhu et al., 2012). Mitochondria are cellular power plants that supply more than 90% of the cellular ATP to support neuronal survival and function, such as axonal growth and branching, generation of action potentials, and synaptic transmission. Mitochondria are also involved in short-term synaptic plasticity and maintain and regulate neurotransmission by buffering presynaptic Ca+2 (Kang et al., 2008; Levy et al., 2003; Tang and Zucker, 1997). Therefore, loss of mitochondria from axonal terminal impairs synaptic transmission likely due to insufficient ATP supply or reduced Ca+2-buffering capacity (Guo et al., 2005; Ma et al., 2009; Stowers et al., 2002).
Neurons are polarized cells with dendrites and a thin long axon that can extend up to 1 meter in motor and sensory neurons. To maintain energy homeostasis throughout the neuron, specialized mechanisms are required to efficiently deliver mitochondria to distal areas where energy supply and Ca+2 buffering capacity are in high demand (Ruthel and Hollenbeck, 2003; Sheng and Cai, 2012). Long-range mitochondrial transport depends upon MT-based motors. The axonal MTs are uniformly polarized, while the dendritic MTs exhibit mixed polarity. The uniform MT polarity has made axons particularly useful for elucidating mechanisms regulating mitochondrial transport: kinesin-1 (KIF5) motors drive anterograde transport distally whereas dynein motors mediate retrograde movement toward the soma. Energy powering motors to drive their cargo transport is from ATP hydrolysis (Hirokawa et al., 2010). Mitochondrial respiration provides the main ATP source, thus powering their own motility (Zala et al., 2013). Both in vitro and in vivo live imaging in different types of neurons consistently reveals a complex motility pattern of mitochondrial transport along axons: mitochondria display bi-directional transport, frequent pause and change in direction, or persistent docking in certain regions. Thus, the mean velocity of neuronal mitochondria is highly variable, ranging from 0.32 to 0.91 um/sec (Macaskill and Kittler, 2010).
In mature neurons, about 20~30% of axonal mitochondria are motile (Chen and Sheng, 2013; Kang et al., 2008); while ~15% mitochondria either briefly pause or dock at synapses; and ~14% motile mitochondria dynamically pass through presynaptic terminals. Our recent study (Sun et al., 2013) demonstrates that an anchored mitochondrion within presynaptic terminals provides a stable and continuous ATP supply. Conversely, in the absence of a mitochondrion within a terminal, there is no stable on-site ATP supply. A motile axonal mitochondrion passing through those terminals temporally supplies ATP, thus changing synaptic energy levels and influencing various ATP-dependent synaptic activities. This study revealed, for the first time, that the fast movement of axonal mitochondria is one of the primary mechanisms underlying the presynaptic variation. This provides new insight into the fundamental properties of the central nervous system to ensure the plasticity and reliability of synaptic transmission.
Axons and synapses are highly plastic and undergo spontaneous and activity-dependent remodeling, thereby changing mitochondrial distribution. In addition, neurons are postmitotic cells surviving for the lifetime of the organism. Aged or dysfunctional mitochondria need to be removed from distal axons. Thus, mitochondria alter their motility under certain pathophysiological stress conditions or when their integrity is impaired (Cai et al., 2012; Miller and Sheetz, 2004). Defective mitochondrial transport and altered distribution are implicated in the pathogenesis of several major neurodegenerative diseases and neurological disorders (Sheng and Cai, 2012). Research into the efficient regulation of mitochondrial trafficking and anchoring in healthy or diseased neurons will advance our knowledge as to how: (1) neurons recruit and redistribute mitochondria to meet altered metabolic requirements; and (2) aged and damaged mitochondria are removed and replenished with healthy ones at distal terminals.
1. Molecular motors driving neuronal mitochondrial transport
Long-range mitochondrial transport between the soma and distal axonal and dendritic terminals are driven by MT-based motor proteins: kinesin superfamily proteins (KIFs) and cytoplasmic dynein. They mediate long-distance transport of mitochondria and other membranous organelles or cargoes through mechanisms that depend on the polarity and organization of neuronal MTs and require ATP hydrolysis (Hirokawa et al., 2010; Vale et al., 1985). Members of the kinesin-1 family (collectively known as KIF5) are the main motors driving plus end-directed anterograde transport of neuronal mitochondria (Hurd and Saxton, 1996; Pilling et al., 2006; Tanaka et al., 1998). There are three isoforms of KIF5 (KIF5A, KIF5B and KIF5C) in mammals. KIF5B is expressed ubiquitously, whereas KIF5A and KIF5C are only found in neurons (Hirokawa et al., 2010). The N-terminus of KIF5 is the motor domain with ATPase and the C-terminal tail is the cargo-binding domain, which links mitochondria via adaptor proteins. Both neuronal imaging and biochemical analyses confirmed that KIF5 motors associate with mitochondria (Hirokawa et al., 1991; Macaskill et al., 2009b; Pilling et al., 2006). Disrupting KIF5-mitochondria coupling in hippocampal neurons impairs mitochondrial transport, thus reducing mitochondrial density in distal axons (Cai et al., 2005). Mutation in Khc, a kinesin heavy chain in Drosophila, disrupts the mitochondrial transport and reduces mitochondrial distribution in larval motor axons (Hurd and Saxton, 1996). Target disruption of KIF5A or KIF5B in mice also impairs mitochondrial transport and results in perinuclear accumulation of mitochondria (Karle et al., 2012; Tanaka et al., 1998; Xia et al., 2003). In addition to KIF5s, two members of the Kinesin-3 family, KIF1B-α and Kinesin-like protein 6 (KLP6), are also involved in regulating mitochondrial transport (Nangaku et al., 1994). Mutant forms of KIF1B-α and KLP6 decrease the mean velocity and density of mitochondria along the axon (Tanaka et al., 2011; Wozniak et al., 2005). Although depleting one of them alters the distribution of mitochondria, their role in driving anterograde mitochondrial transport in axons requires further characterization.
Cytoplasmic dynein is the major motor drives MT-based retrograde transport in axons. It contains multiple subunits including two catalytic heavy chains (DHC), several intermediate (DIC), light intermediate (DLIC) and light chains (DLC), which mediate cargo binding or regulate motor activity. The C-terminus of DHC is the motor domain required for dynein movement. Dynactin, a large protein complex, binds directly to dynein and MTs through its p150Glued subunit. Both dynein and dynactin associate with mitochondria and are essential for driving mitochondria retrograde transport in D. melanogaster (Pilling et al., 2006). Mutation in dynein reduces mitochondrial retrograde run length and duration in fly motor neurons. Interestingly, disruption of the dynactin complex does not disrupt the attachment of motor proteins to membranes but interrupts both anterograde and retrograde transport, which suggests that dynactin is involved in regulating both KIF5- and dynein-driven bi-directional transport (Haghnia et al., 2007). These opposing kinesin and dynein motors were localized on the same mitochondrion, highlighting the complex mobility patterns of axonal mitochondria. As axonal mitochondria exhibit bidirectional movement and dynein can colocalize with mitochondria moving in either direction (Hirokawa et al., 1990), it is likely that kinesin and dynein coordinate the transport of individual mitochondria. Future investigations are necessary to determine to what extent regulatory crosstalk between kinesin and dynein coordinates the distribution and motility state of axonal mitochondria in response to metabolic changes and synaptic activity.
2. Motor adaptors regulating mitochondrial transport
Mitochondria recruit motors by indirectly associating with their respective motor adaptor proteins and mitochondrial membrane receptors (Figure 1A). These motor/adaptor/receptor complexes ensure targeted mitochondrial trafficking and precise regulation of their distribution in response to changes in neuronal activity. The Drosophila protein Milton is the first identified adaptor that links the mitochondrial outer membrane protein Miro (as a receptor) to the KIF5 cargo-binding domain (Glater et al., 2006). There are two mammalian Milton orthologues, TRAK1 and TRAK2, respectively (Koutsopoulos et al., 2010; Macaskill et al., 2009b). The milton mutant in Drosophila results in mitochondria loss at synaptic and axonal terminals (Stowers et al., 2002). Depleting TRAK1, or expressing its dominant-negative mutants in hippocampal neurons impairs axonal mitochondrial motility (Brickley and Stephenson, 2011; van Spronsen et al., 2013). Conversely, overexpression of TRAK2 robustly enhances mitochondrial motility (Chen and Sheng, 2013). TRAK1 and TRAK2 may have different roles in regulating mitochondrial motility in axons versus dendrites (van Spronsen et al., 2013). TRAK1 binds to both KIF5 and dynein and steers mitochondria into axons, whereas TRAK2 predominantly interacts with dynein/dynacitn and mediates dendritic targeting. The conformational change induced by the head-to-tail binding of TRAK2 disrupts its interaction with kinesin-1, thus allowing Trak2-dynein complex transport into dendrites. This study suggests that TRAK1/2 may coordinate KIF5- and dynein-driven bidirectional transport and polarized targeting (Figure 1A) (Franker and Hoogenraad, 2013).
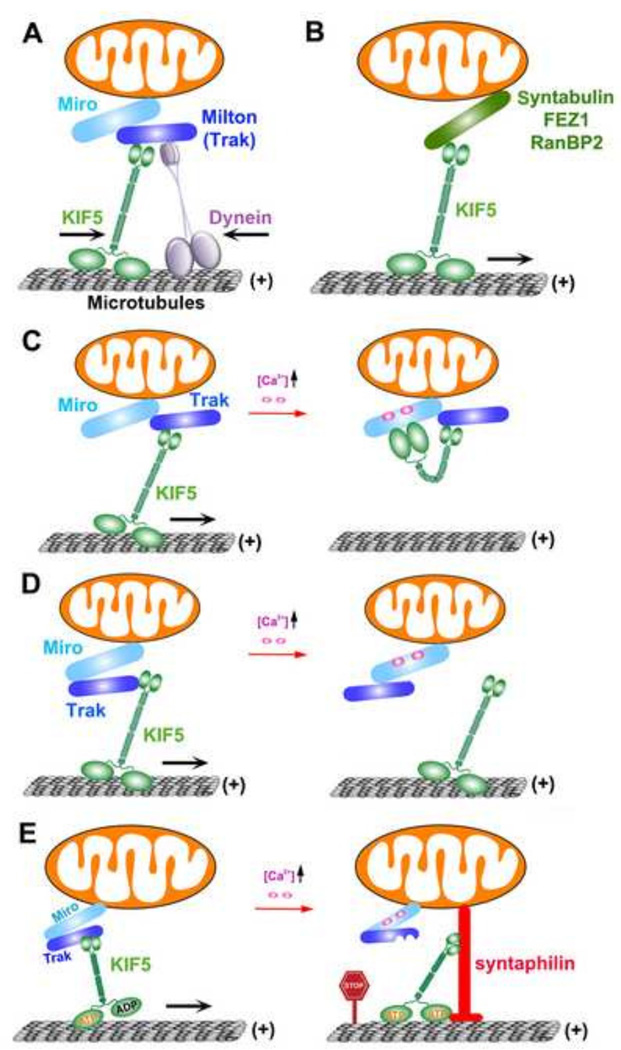
Figure 1. Regulation of mitochondrial transport by motor-adaptor complexes and docking receptor.
(A). The Miro-Milton (Miro-TRAK) complexes link KIF5 motors for driving mitochondrial transport (Glater et al., 2006; (Koutsopoulos et al., 2010; Macaskill et al., 2009a). Miro and TRAK may also serve as a receptor for dynein for mediating retrograde transport (Franker and Hoogenraad, 2013; Russo et al., 2009; Nguyen et al., 2014; Russo et al., 2009). Thus, relative motility of the opposite motors can be coordinated by mitochondrial adaptor/receptor complexes.
(B) Syntabulin serves as an alternative KIF5 motor adaptor for driving mitochondrial anterograde transport (Cai et al., 2005).
(C, D) Miro-Ca2+ sensing models for activity-dependent regulation of mitochondrial motility. The C-terminal cargo-binding domain of KIF5 motors binds to the Miro-TRAK adaptor complex. Ca2+ binding to Miro’s EF-hands induces the motor domain to disconnect with MTs and thus prevents motor–MT engagement (C) (Wang and Schwarz, 2009). Alternatively, Ca2+ binding releases KIF5 motors from mitochondria (D) (Macaskill et al., 2009b). Thus, Ca2+ influx upon synaptic activity arrests motile mitochondria at activated synapses.
(E) Syntaphilin-mediated “engine-switch and brake” model. A Miro-Ca2+ sensing pathway triggers the binding switch of KIF5 motors from the Miro-TRAK adaptor complex to docking receptor syntaphilin, which immobilizes axonal mitochondria via inhibiting motor ATPase activity. Thus, syntaphilin turns off the “Engine” (KIF5 motor) by sensing a “Stop Sign” (elevated Ca2+) and putting a brake on mitochondria (Chen and Sheng, 2013). Figure is modified from Sheng (2014) originally published in Journal of Cell Biology, 204, 1087–1098 (doi/10.1083/jcb.201312123)
Miro is a member of the mitochondrial outer membrane Rho GTPase family with four Ca+2-binding EF-hand motifs and two GTPase domains (Fransson et al., 2006; Frederick et al., 2004; Klosowiak et al., 2013). It functions as a mitochondrial receptor by binding the motor adaptor Milton (or TRAK1/2), thereby recruiting KIF5 motors to the mitochondrial surface (Figure 1A). dMiro mutant impairs mitochondrial anterograde transport to distal regions, thus depleting axonal and synaptic mitochondria (Guo et al., 2005). Mammals have two Miro orthologs, Miro-1 and Miro-2, which share 60% sequence identity. Elevated Miro1 expression increases mitochondrial transport by recruiting more TRAK and motors to mitochondria (Chen and Sheng, 2013; Macaskill et al., 2009b). Emerging lines of evidence suggest that Miro may also serve as a receptor for dynein. Drosophila dMiro helps regulate both anterograde and retrograde transport of axonal mitochondria (Russo et al., 2009). dmiro null mutant (Russo et al., 2009) or loss of Miro-1 in mouse cortical neurons (Nguyen et al., 2014) impairs retrograde mitochondrial transport. Interestingly, neuron-specific loss of Miro1 causes depletion of mitochondria from corticospinal tract axons and progressive neurological deficits mirroring human upper motor neuron disease. The Milton/Miro complex is formed with dynein, thus highlighting a new model in which the relative motility of the opposite motors can be coordinated by the mitochondrial adaptor/receptor complex. An in-depth review of Miro-mediated regulation of mitochondrial transport can be found in an accompanying review article of the same issue.
Syntabulin is the second KIF5 motor adaptor that contains a mitochondrion-targeted carboxyl-terminal transmembrane domain. Unlike Miro which links the KIF5 motor via binding the motor adaptor Milton (TRAK1/2), syntabulin directly interacts with the cargo-binding domain of KIF5, thus recruiting KIF5 motors to mitochondria (Cai et al., 2005; Su et al., 2004). In cultured hippocampal neurons, knockdown of syntabulin or inhibition of syntabulin-KIF5 coupling by expressing KIF5-binding domain transgenes results in mitochondrial clustering in the soma and reduced mitochondrial distribution at distal processes. Similarly, mobility analyses in live neurons demonstrate that syntabulin loss-of-function reduces anterograde, but not retrograde, transport of mitochondria along axons. These phenotypes support a role for syntabulin as a KIF5 motor adaptor mediating mitochondrial anterograde transport (Figure 1B).
Several other proteins were also suggested as adaptors that connect KIF5 to mitochondria. For example, silencing fasciculation and elongation protein-1 (FEZ-1), which is involved in NGFinduced neurite outgrowth, mediates the mitochondrial anterograde transport along hippocampal axons and neurites of PC12 cells (Fujita et al., 2007). The association between FEZ1 and c-Jun N-terminal kinase (JNK)-interacting protein (JIP1) can activate kinesin motor activity (Blasius et al., 2007). Another candidate adaptor is RNA-binding protein 2 (RanBP2), which co-localizes and interacts with KIF5B and KIF5C and regulates mitochondrial transport in non-neuronal cells (Cho et al., 2007; Patil et al., 2013). Thus, the existence of multiple motor adaptors may reflect the complex regulation of mitochondrial motility in different neurons in response to various physiological and pathological signals.
3. Anchoring receptor immobilizing axonal mitochondria
ATP has a limited diffusion capacity, particularly within long axonal process; thus, anchored mitochondria ideally serve as local energy power plants. In mature neurons, approximately 30% of axonal mitochondria move bi-directionally, some of which pass through or pause at presynaptic terminals. Motile mitochondria become stationary and stationary ones are remobilized and re-distributed. The balance between motile and stationary pools of mitochondria responds quickly to changes in axonal physiology and synaptic activity. Dissociation of mitochondria from motors or their anchoring to the cytoskeleton was proposed to recruit motile mitochondria into the stationary pools along axons. Efficient regulation of mitochondrial motility is crucial to ensure that metabolically active areas are adequately supplied with ATP. For example, synaptic transmission is regulated by local mitochondria anchored at presynaptic terminals (Kang et al., 2008). In addition, mitochondrial docking is required for axonal branching and maintenance (Courchet et al., 2013).
Syntaphilin is one intriguing mitochondrial docking protein, which acts as a “static anchor” immobilizing mitochondria specifically in axons (Chen et al., 2009; Chen and Sheng, 2013; Kang et al., 2008). Syntaphilin is an axon-targeted mitochondrial outer membrane protein through its axon-sorting sequence and carboxyl-terminal mitochondria-targeting domain. Syntaphilin arrests mitochondrial movement by anchoring them to MTs. Deleting syntaphilin in mice results in a robust increase of axonal mitochondria in motile pools, thus reducing mitochondrial density within the axons. Conversely, over-expressing syntaphilin abolishes axonal mitochondrial transport. Thus, syntaphilin ensures that neurons maintain the proper mitochondrial density within axons and at synapses. Syntaphilin, therefore, serves as an attractive molecular target for investigations into mechanisms recruiting motile mitochondria to activated synapses. The syntaphilin mouse is an ideal genetic model to examine how axonal mitochondrial anchoring impacts presynaptic function and mitochondrial quality control in healthy neurons, and pathological progression in mouse models of neurodegenerative diseases.
4. Synaptic activity-dependent regulation of mitochondrial transport
The distribution of mitochondria is highly correlated with energy demand. Stationary mitochondria usually locate at the site with high-energy demand, such as synapses. Mitochondrial transport in axons and distribution at synapses is correlated with synaptic activity. Mitochondria are recruited to synapses in response to elevated intracellular Ca2+, either by activating voltage-dependent calcium channels at presynaptic terminals or NMDA receptors at postsynaptic sites (Chang et al., 2006; Rintoul et al., 2006; Szabadkai et al., 2006; Yi et al., 2004). The mechanisms by which mitochondria are recruited to the stationary pool and arrested at synapses during sustained synaptic activity were the main subject of studies in several laboratories during the past decade. Recent studies of KIF5-TRAK-Miro complexes are revealing how synaptic Ca2+ levels regulate mitochondrial trafficking and anchoring (Sheng, 2014).
Miro serves as a Ca2+ sensor in regulating mitochondrial motility. Elevated Ca2+ at activated synapses binds to the EF-hands of Miro. This Ca2+-binding induces the conformational changes either inactivating or disassembling the KIF5-Miro-TRAK transport machineries, thus immobilizing mitochondria at active synapses (Macaskill et al., 2009a; Saotome et al., 2008; Wang and Schwarz, 2009). Based on these studies, two alternative Miro-Ca2+ sensing models were proposed: at resting Ca2+ levels, KIF5 motors are loaded onto mitochondria through physical coupling with the motor adaptor complex Miro-Milton (Drosophila) or Miro-TRAK1/2 (mammals), while the N-terminal motor domain remains engaging MTs for driving anterograde transport. This Ca2+-sensing pathway induces a direct interaction between KIF5 motor domain and Miro, thus preventing KIF5-MTs engagement and arresting the mitochondria at active synapses (Figure 1C) (Wang and Schwarz, 2009). Alternatively, Macaskill et al (2009b) proposed that Ca+2 binding to Miro detaches KIF5 motors from mitochondria and therefore, mitochondria passing by active synapses are immobilized (Figure 1D). Given the fact that knockdown or depletion of Miro1 in cell lines or primary cortical neurons does not abolish Ca+2-induced mitochondrial immobilization (Nguyen et al., 2014; Saotome et al., 2008), an important question remains if Miro2 is the predominant Ca+2-sensor for activity-dependent regulation of mitochondrial transport. In addition, the Miro-Ca2+ pathway affects both anterograde and retrograde transport. When KIF5 transport machinery is disrupted by the Miro-Ca2+-sensing mechanism, dynein-driven retrograde transport does not necessarily take over, raising the possibility that the Miro-Ca2+ pathway immobilizes mitochondria depending on an anchoring mechanism.
Our recent study demonstrates that syntaphilin is required for activity-dependent immobilization of axonal mitochondria (Chen and Sheng, 2013). Using syntaphilin-null mouse models and time-lapse imaging analysis in live hippocampal neurons, we provide convincing evidence to support the above hypothesis. Activating the Miro-Ca2+ pathway by firing neurons with KCl treatment or electrical field stimulation, fails to arrest axonal mitochondria in syntaphilin−/− neurons. Axonal mitochondria keep moving during neuronal firing while dendritic mitochondria are effectively immobilized, suggesting that syntaphilin plays a central role in the activity-dependent immobilization of axonal mitochondria. Syntaphilin specifically binds the KIF5 C-terminal tail through its KIF5-binding domain (KBD). Interfering with the KIF5-syntaphilin interaction in neurons by expressing KBD abolishes activity-dependent regulation of axonal mitochondrial transport. Interestingly, Miro-Ca2+ sensing facilitates KIF5-syntaphilin interaction that inhibits motor ATPase activity and reduces assembly of the Miro1-TRAK2-KIF5 transport complex. In addition, syntaphilin is recruited to axonal mitochondria following sustained neuronal activity, suggesting that syntaphilin undergoes dynamic translocation to axonal mitochondria in response to neuronal activity, although the mechanism underlying such translocation remains unknown.
This study provides new mechanistic insights into the activity-dependent anchoring of axonal mitochondria at synapses. Miro-Ca2+ sensing releases the carboxyl tail of KIF5 from the Miro-TRAK motor adaptor complex for binding to syntaphilin. This binding shift results in inhibition of the motor ATPase. Thus, syntaphilin arrests axonal mitochondrial movement by: (1) interfering with the motor transport complex; and (2) inactivating the motor ATPase. We propose a new “engine-switch and brake” model (Figure 1E). In response to a ‘stop’ sign (elevated Ca2+) at active synapses, Syntaphilin switches off KIF5 motors and puts a brake on mitochondria. This model may help reconcile the standing disagreements regarding how Miro-Ca2+ sensing immobilizes mitochondria in dendrites versus axons. KIF5 motors remain associated with immobilized mitochondria within axons during elevated Ca2+ conditions (Figure 1C) (Wang and Schwarz, 2009), or KIF5 motors disconnect from immobilized mitochondria in dendrites after activating glutamate receptors (Figure 1D) (Macaskill et al., 2009b). Our study suggests that upon Miro-Ca2+ sensing, KIF5 motors remain associated with axonal mitochondria by binding syntaphilin. This KIF5-syntaphilin coupling may facilitate axonal mitochondria being quickly moved away to new active synapses once the Ca2+ signal is removed. This new model also suggests that motor loading is insufficient for driving cargo transport. Turning off the anchoring switch is also required and this is consistent with multiple observations that both KIF5 and dynein motors remain bound on cargoes regardless of whether they are motile or stationary.
5. Metabolic signaling-mediated regulation of mitochondrial transport
Efficient recruitment and retention of mitochondria as local energy sources is crucial to ensure that metabolically active areas are adequately supplied with ATP. Depletion of local ATP via glutamate application, for example, reduces local mitochondrial transport velocity. In contrast, elevated ADP levels due to increased ATP consumption recruit mitochondria to synapses (Mironov, 2007). However, mechanisms coordinating mitochondria transport by sensing energy consumption are unclear. Recent studies support the notion that recruitment of motile mitochondria into stationary pools in axons responds quickly to changes in axonal growth status via AMPK, an AMP-activated protein kinase. AMPK is a master regulator of cellular energy homeostasis and is activated in response to stresses that deplete cellular ATP supplies. Formation and stabilization of axonal branching depend upon the local ATP supply to support cytoskeleton reorganization, localized protein synthesis, and axonal transport (Spillane et al., 2013). Activation of AMPK increases anterograde flux of mitochondria into axons and induces axonal branching in regions where mitochondria are docked in an ATP-dependent manner (Tao et al., 2014). Arresting mitochondrial motility at axon branching points occurs by activating LKB1, the serine/threonine liver kinase B1, and its downstream AMPK-like kinase NUAK1. Deleting LKB1 or NUAK1 reduces stationary pools of axonal mitochondria, while expressing LKB1 or NUAK1 immobilizes mitochondria (Courchet et al., 2013). Intriguingly, suppressing syntaphilin reduces axon branching while expressing syntaphilin increases axon branching, thus establishing a causal correlation between syntaphilin-mediated mitochondrial anchoring and LKB1-AMPK-induced axonal branching. However, an important mechanistic question remains: Does syntaphilin act as a downstream effector of AMPK pathways in recruiting mitochondria by sensing metabolic signals? Addressing this issue seems directly relevant to the challenge neurons have in maintaining energy supply in neurological disorders. This is supported by a recent work on demyelinated axons, a major cause of neurological disability in primary diseases of myelin. Syntaphilin-mediated anchoring is required for the increased mitochondria volume in demyelinated regions, thus protecting against axonal degeneration (Ohno et al., 2014). This study suggests that, in addition to myelin loss, impaired anchoring of axonal mitochondria may contribute to degeneration of demyelinated axons.
A recent study shows that glucose levels could regulate neuronal mitochondrial motility by O-GlcNAc transferase (OGT) (Pekkurnaz et al., 2014). OGT-mediated O-GlcNAcylation, which attaches a single sugar moiety to the serine or threonine residue of Milton, has been shown in fly and mammalian cells. In response to elevated extracellular glucose, OGT induces the O-GlcNAcylation of Milton, resulting in immobilization of mitochondria. Interestingly, O-GlcNacylation dose not disrupt the KIF5-Milton complex, leaving a mechanistic question as how the O-GlcNAcylation event regulates mitochondrial transport machinery. In addition to the metabolic states and Ca2+, nerve growth factor (NGF), neurotransmitters 5-HT1A and nitric oxide (NO) also serve as docking signals that immobilize axonal mitochondria. NGF and 5- HT1A immobilize mitochondria via the downstream PI3K and Akt/GSK3β pathways, respectively (Chen et al., 2008; Morris and Hollenbeck, 1995; Sang et al., 2001). Application of an NO donor, such as PAPA-NO, immobilizes mitochondria by inhibiting respiration and ATP synthesis (Rintoul et al., 2006; Zanelli and Trimmer, 2006). These findings may support a recent study that motor-driven mitochondrial transport relies on energy from the respiration reaction (Zala et al., 2013).
Mitochondrial integrity impacts their transport
Throughout a neuron's lifetime, aged and damaged mitochondria undergo a variety of quality control mechanisms to ensure their integrity, such as fusion-fission dynamics and/or degradation via mitophagy, a cargo-specific autophagy-lysosomal pathway (Chen and Chan, 2009; Sheng and Cai, 2012). Mitochondrial dysfunction, accompanied by defective transport, is a key hallmark of age-associated neurodegenerative diseases. Dysfunctional mitochondria are not only less efficient in producing ATP but also release harmful reactive oxygen species. The proper sequestration of damaged mitochondria and subsequent degradation through the lysosomal system may serve as an early neuroprotective mechanism. Mature acidic lysosomes are mainly located in the soma. Thus, a fundamental question remains: How are damaged mitochondria at distal terminals efficiently eliminated? Investigations into how mitochondrial motility coordinates removal of damaged mitochondria from axonal terminals emerge as a central area in neurobiology.
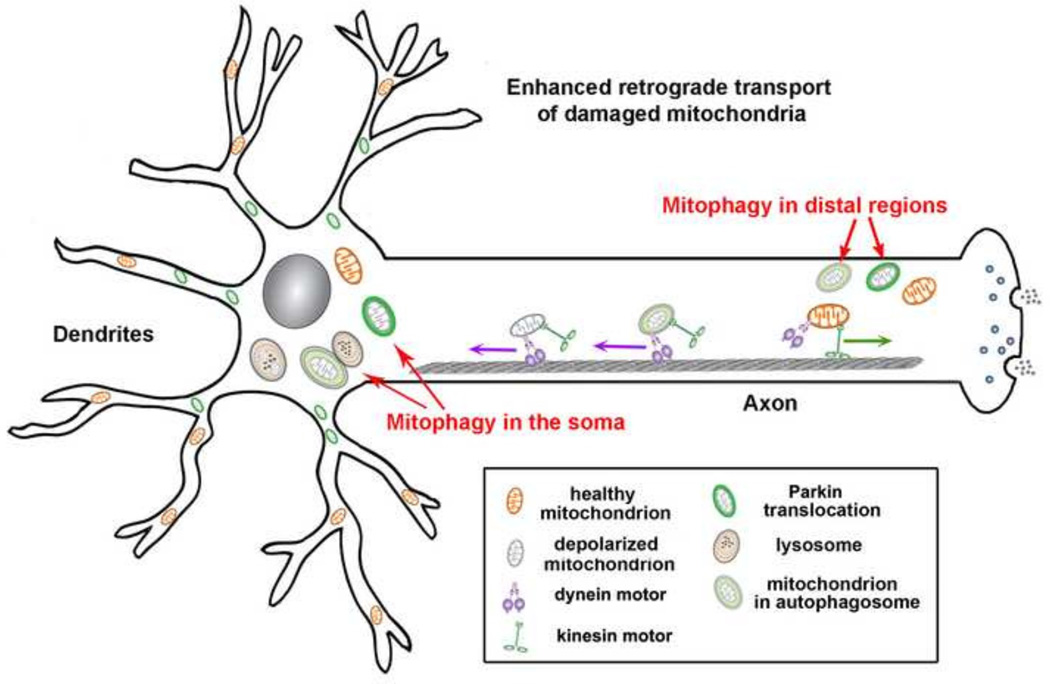
Cumulative evidence revealed that PTEN-induced putative kinase protein 1 (PINK1) and Parkin, a cytosolic E3 ubiquitin ligase, play critical roles in removing damaged mitochondria. When mitochondrial integrity is damaged or their membrane potential (Δψ) is reduced, PINK1 accumulates on the outer mitochondrial membrane and recruits Parkin from the cytosol. Parkin ubiquitinates mitochondrial proteins, a process essential for damaged mitochondria to be engulfed by isolation membranes and then for degradation by lysosomes. In addition to Parkinmediated mitophagy, other mitochondrial E3 ligases, such as Mul1, may maintain mitochondrial integrity in parallel to the Pink/Parkin pathway (Yun et al., 2014). However, evidence showing Parkin-mediated mitophagy in mature neurons is controversial (Sheng and Cai, 2012), leaving unanswered questions as to how and where Parkin-mediated mitophagy occurs in neurons. Given inconsistent observations in the literature, we speculate that the quality of primary cultured neurons is critical to examine dynamic mitochondrial transport and Parkin translocation following dissipation of mitochondrial membrane potential (Δψm). We recently established high-quality mature cortical neuronal cultures, which survive long enough to exhibit Parkin-mediated mitophagy and altered mitochondrial transport (Cai et al., 2012). Under these conditions, neurons survive long enough to exhibit much slower and milder Parkin-mediated mitophagy following chronic Δψm dissipation for 24 hours. Such chronic stress conditions allow detection of the altered mitochondrial transport, thus better reflecting mitochondria stress under in vivo pathophysiological conditions. Our study reveals three unique features for Parkin-mediated mitophagy in mature neurons: (1) Parkin-mediated mitophagy is a much slower and milder process than in non-neuronal cells and only occurs in a small portion of neurons; (2) Intriguingly, Parkin-targeted mitochondria are predominantly found in the soma and proximal region of processes following chronically dissipating Δψm with low concentrations of uncoupling reagents; (3) Such compartmental restriction is due to altered motility of depolarized mitochondria with reduced anterograde and enhanced retrograde transport, thus reducing anterograde flux of damaged mitochondria into distal processes (Cai et al., 2012). The correlation between mitochondrial Δψm and motility was also observed in a previous study by acutely treating neurons with high concentrations of dissipating reagents (Miller and Sheetz, 2004; Verburg and Hollenbeck, 2008). Altered motility may be protective under chronic mitochondrial stress conditions; thus, healthy mitochondria remain distally while aged and damaged ones return to the soma for degradation, where mature acidic lysosomes are relatively enriched (Figure 2).
Figure 2. Mitochondrial integrity impacts their transport.
Chronically depolarized mitochondria, by dissipating mitochondrial membrane potential (Δψm) with uncoupling reagents alters motility with reduced anterograde and enhanced retrograde transport, thus resulting in accumulation of Parkin-targeted mitochondria in the soma and proximal regions (Cai et al., 2012). This spatial process allows neurons to efficiently remove dysfunctional mitochondria from distal axons to the soma where mature acidic lysosomes are relatively enriched. Damaged mitochondria at axonal terminals can also recruit Parkin for mitophagy once they are anchored by syntaphilin (Cai et al., 2012) or immobilized by turnover of the motor adaptor Miro on the mitochondrial surface (Ashrafi et al, 2014). Autophagosomes engulfing damaged mitochondria at axonal terminals transport predominantly to the soma for maturation and degradation within acidic lysosomes (Maday et al., 2012). Figure is modified from Sheng (2014) originally published in Journal of Cell Biology, 204, 1087–1098 (doi/10.1083/jcb.201312123).
However, damaged mitochondria at terminals can also recruit Parkin for mitophagy once they are anchored by overexpressing the mitochondrial docking protein syntaphilin (Cai et al., 2012) or immobilized by degradation of Miro (Chan et al., 2011; Liu et al., 2012; Wang et al., 2011; Yoshii et al., 2011). Parkin mediates degradation of Miro on the mitochondrial surface upon Δψm dissipation (Sarraf et al., 2013; Weihofen et al., 2009). Interestingly, Miro also interacts with PINK1 and Parkin and is ubiquitinated by Parkin once mitochondria are depolarized. Turnover of Miro on the mitochondrial surface may favor their retrograde transport to the soma. Given the fact that (1) a small population of mito-autophagosomes is labeled with late endosome/lysosomal marker LAMP1 (Ashrafi et al., 2014); and (2) retrograde transport of autophagosomes is essential for maturation and degradation within acidic lysosomes in the proximal region of the neuron (Maday et al., 2012), distal mitophagy may not represent an efficient degradation pathway in removing damaged mitochondria through the neuronal lysosomal system. Thus, a functional interplay is proposed between mitochondrial motility and mitophagy to ensure efficient removal of dysfunctional mitochondria from distal processes. Future investigations into mechanisms coordinating mitochondrial retrograde transport and quality control will advance our understanding of human neurodegeneration.
Summary
Recent studies provided insight into the regulation of mitochondrial trafficking and anchoring in response to changes in neuronal activity, metabolic signaling, and mitochondrial integrity (Sheng, 2014). However, there are mechanistic questions to be addressed. For example, how does the Miro-Ca+2-sensing pathway inactivate both anterograde and retrograde transport? Does Ca+2 sensing inactivate dynein motor activity or release it from mitochondria? Why do neurons need multiple adaptors for mitochondrial transport and how do they decide which adaptor to attach for motor-driven transport? In particular, it will be important to investigate how mitochondria coordinate the balance between motile and stationary pools in sensing mitochondrial membrane potential, cellular metabolic status, neuronal development, and pathological stress. Studying these dynamic cellular processes in live adult neurons, rather than embryonic neurons, from genetic or disease mouse models will advance our understanding of aging-associated neurodegenerative diseases.
Acknowledgments
The authors thank all the colleagues in their laboratory and other laboratories who contributed to the research described in this article. The authors’ lab is supported by the Intramural Research Program of NINDS, NIH (Z-H. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests statement
The authors declare no competing financial interests.
References
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014;98:15294. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J. Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J. Biol. Chem. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gerwin C, Sheng Z-H. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J. Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Zakaria HM, Simone A, Sheng Z-H. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr. Biol. 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DTW, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Owens GC, Edelman DB. Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS ONE. 2008;3:e2804. doi: 10.1371/journal.pone.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sheng Z-H. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J. Cell Biol. 2013;202:351–364. doi: 10.1083/jcb.201302040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-M, Gerwin C, Sheng Z-H. Dynein light chain LC8 regulates syntaphilinmediated mitochondrial docking in axons. J. Neurosci. 2009;29:9429–9438. doi: 10.1523/JNEUROSCI.1472-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K-I, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA. Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function. Traffic. 2007;8:1722–1735. doi: 10.1111/j.1600-0854.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Lee S, Courchet V, Liou D-Y, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franker MAM, Hoogenraad CC. Microtubule-based transport - basic mechanisms, traffic rules and role in neurological pathogenesis. J. Cell Sci. 2013;126:2319–2329. doi: 10.1242/jcs.115030. [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J. Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Maturana AD, Ikuta J, Hamada J, Walchli S, Suzuki T, Sawa H, Wooten MW, Okajima T, Tatematsu K, Tanizawa K, Kuroda S. Axonal guidance protein FEZ1 associates with tubulin and kinesin motor protein to transport mitochondria in neurites of NGF-stimulated PC12 cells. Biochem. Biophys. Res. Commun. 2007;361:605–610. doi: 10.1016/j.bbrc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Haghnia M, Cavalli V, Shah SB, Schimmelpfeng K, Brusch R, Yang G, Herrera C, Pilling A, Goldstein LSB. Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol. Biol. Cell. 2007;18:2081–2089. doi: 10.1091/mbc.E06-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J. Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J. Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-S, Tian J-H, Pan P-Y, Zald P, Li C, Deng C, Sheng Z-H. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle KN, Möckel D, Reid E, Schöls L. Axonal transport deficit in a KIF5A(−/−) mouse model. Neurogenetics. 2012;13:169–179. doi: 10.1007/s10048-012-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosowiak JL, Focia PJ, Chakravarthy S, Landahl EC, Freymann DM, Rice SE. Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO Rep. 2013;14:968–974. doi: 10.1038/embor.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos OS, Laine D, Osellame L, Chudakov DM, Parton RG, Frazier AE, Ryan MT. Human Miltons associate with mitochondria and induce microtubule-dependent remodeling of mitochondrial networks. Biochim. Biophys. Acta. 2010;1803:564–574. doi: 10.1016/j.bbamcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Cai Q, Lu W, Sheng Z-H, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J. Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009a;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill AF, Brickley K, Stephenson FA, Kittler JT. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell. Neurosci. 2009b;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur ELF. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Mironov SL. ADP regulates movements of mitochondria in neurons. Biophys. J. 2007;92:2944–2952. doi: 10.1529/biophysj.106.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler M-H, Smith NK, Macfarlane J, Saunders G, Palmer CA, Debattisti V, Koshiba T, Pulst S, Feldman EL, Hajnóczky G, Shaw JM. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E3631–E3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Chiang H, Mahad DJ, Kidd GJ, Liu L, Ransohoff RM, Sheng Z-H, Komuro H, Trapp BD. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9953–9958. doi: 10.1073/pnas.1401155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H, Cho K-I, Lee J, Yang Y, Orry A, Ferreira PA. Kinesin-1 and mitochondrial motility control by discrimination of structurally equivalent but distinct subdomains in Ran-GTP-binding domains of Ran-binding protein 2. Open Biol. 2013;3:120183. doi: 10.1098/rsob.120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158:54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Bennett VJ, Papaconstandinou NA, Reynolds IJ. Nitric oxide inhibits mitochondrial movement in forebrain neurons associated with disruption of mitochondrial membrane potential. J. Neurochem. 2006;97:800–806. doi: 10.1111/j.1471-4159.2006.03788.x. [DOI] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J. Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang H, Lu Z, Li Y, Ru B, Wang W, Chen J. Phosphorylation of tau by glycogen synthase kinase 3beta in intact mammalian cells influences the stability of microtubules. Neurosci. Lett. 2001;312:141–144. doi: 10.1016/s0304-3940(01)02206-6. [DOI] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J. Cell Biol. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Su Q, Cai Q, Gerwin C, Smith CL, Sheng Z-H. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat. Cell Biol. 2004;6:941–953. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan P-Y, Chen Y, Sheng Z-H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim. Biophys. Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sugiura Y, Ichishita R, Mihara K, Oka T. KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells. J. Cell Sci. 2011;124:2457–2465. doi: 10.1242/jcs.086470. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka, Sen Takeda S, Harada A, Hirokawa N. Targeted Disruption of Mouse Conventional Kinesin Heavy Chainkif5B, Results in Abnormal Perinuclear Clustering of Mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev. Neurobiol. 2014;74:557–573. doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J. Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesinmediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-H, Roberts EA, Her L-S, Liu X, Williams DS, Cleveland DW, Goldstein LSB. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii SR, Kishi C, Ishihara N, Mizushima N. Parkin mediates proteasomedependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 2011;286:19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Puri R, Yang H, Lizzio MA, Wu C, Sheng Z-H, Guo M. MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife. 2014;3:e01958. doi: 10.7554/eLife.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Hinckelmann M-V, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Trimmer PA, Solenski NJ. Nitric oxide impairs mitochondrial movement in cortical neurons during hypoxia. J. Neurochem. 2006;97:724–736. doi: 10.1111/j.1471-4159.2006.03767.x. [DOI] [PubMed] [Google Scholar]
- Zhu X-H, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60:2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]