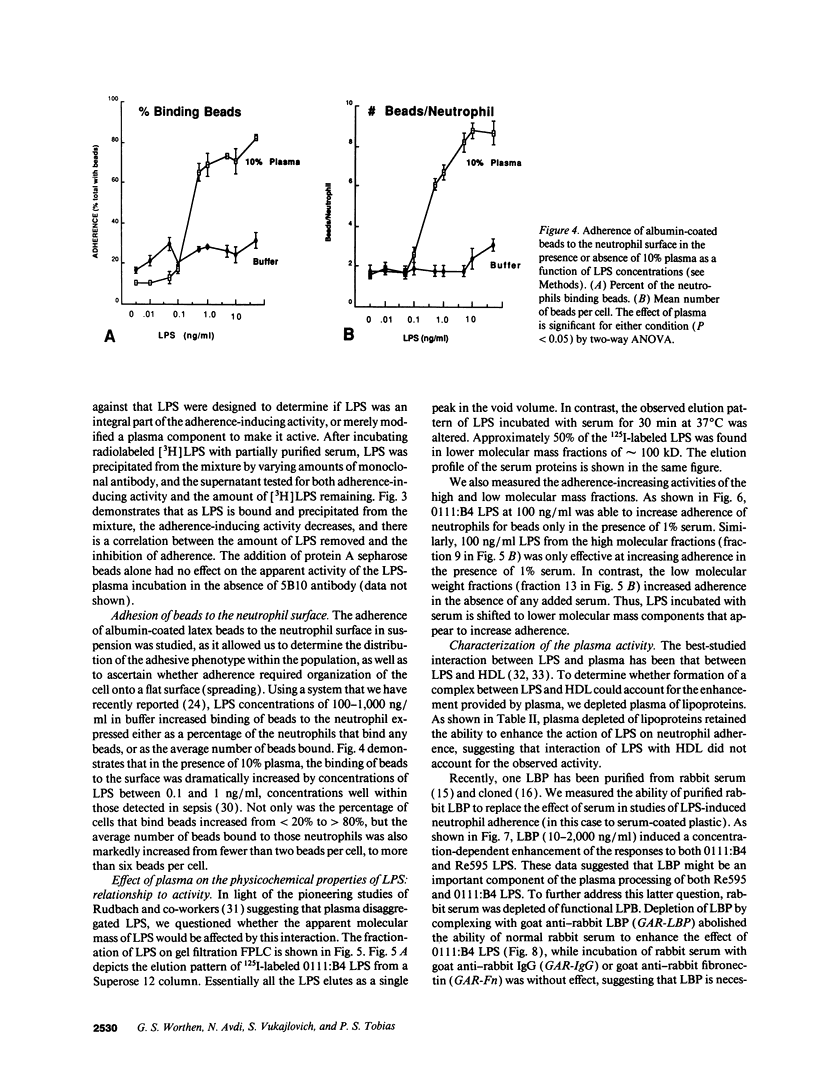
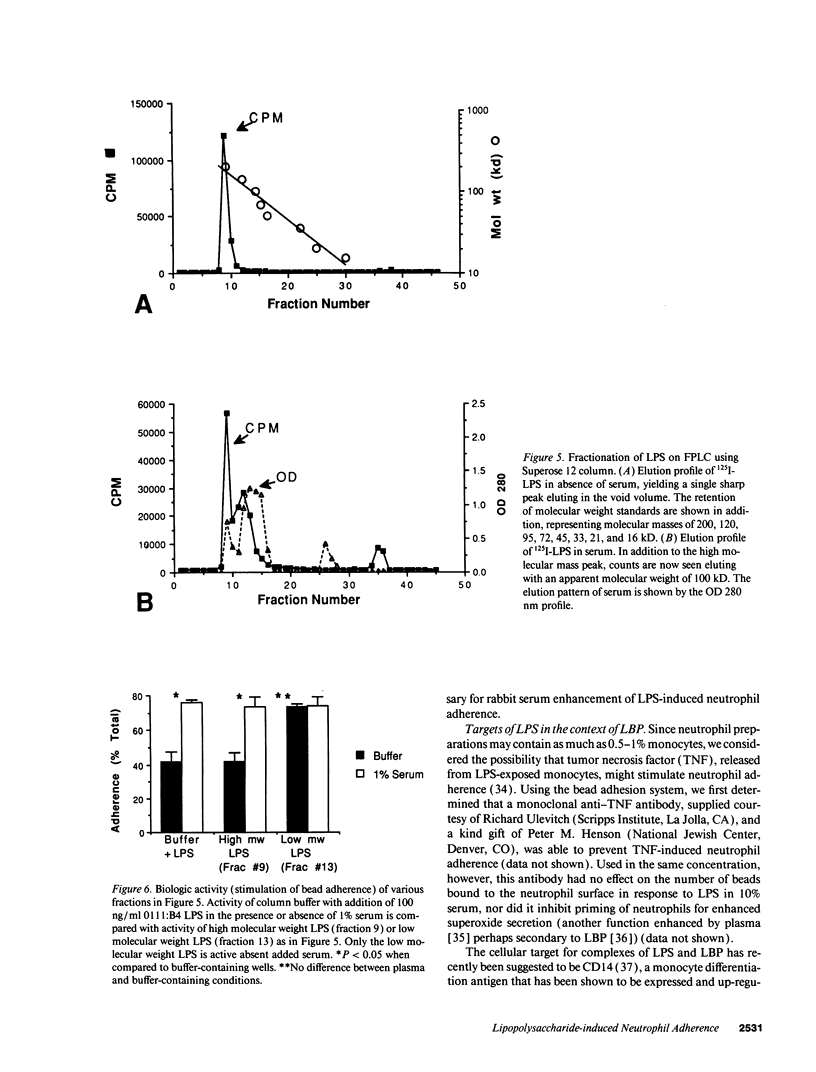
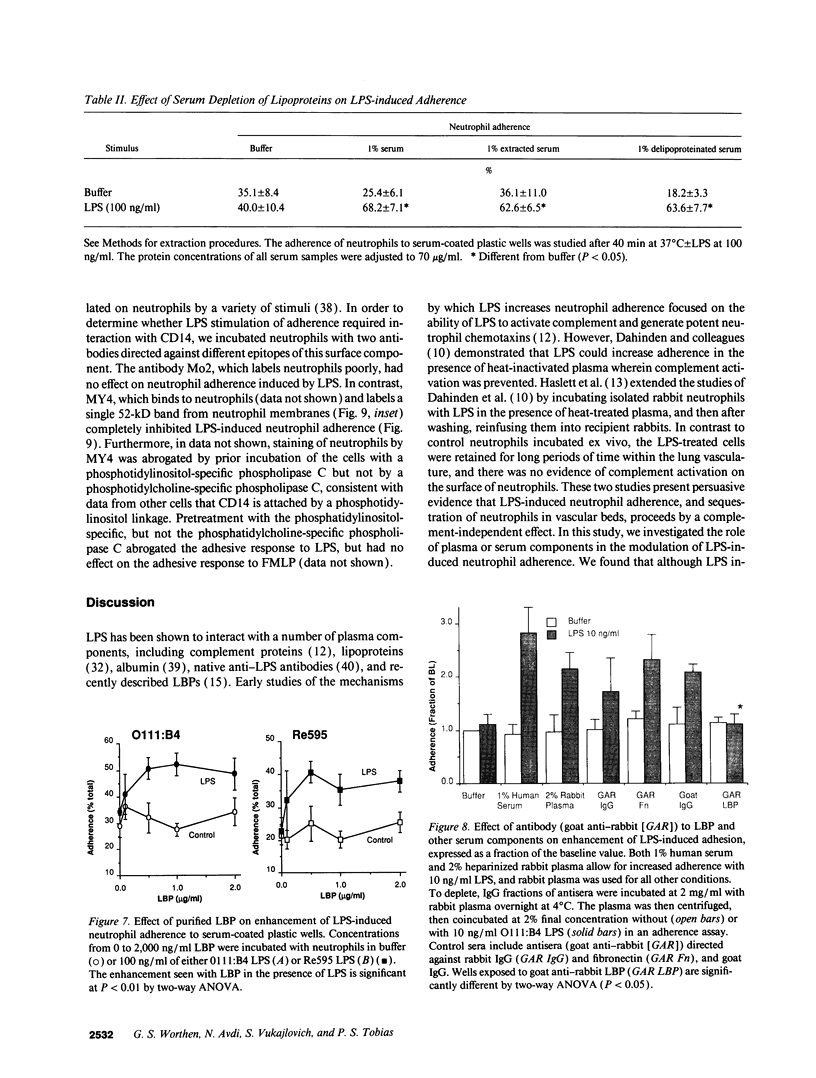
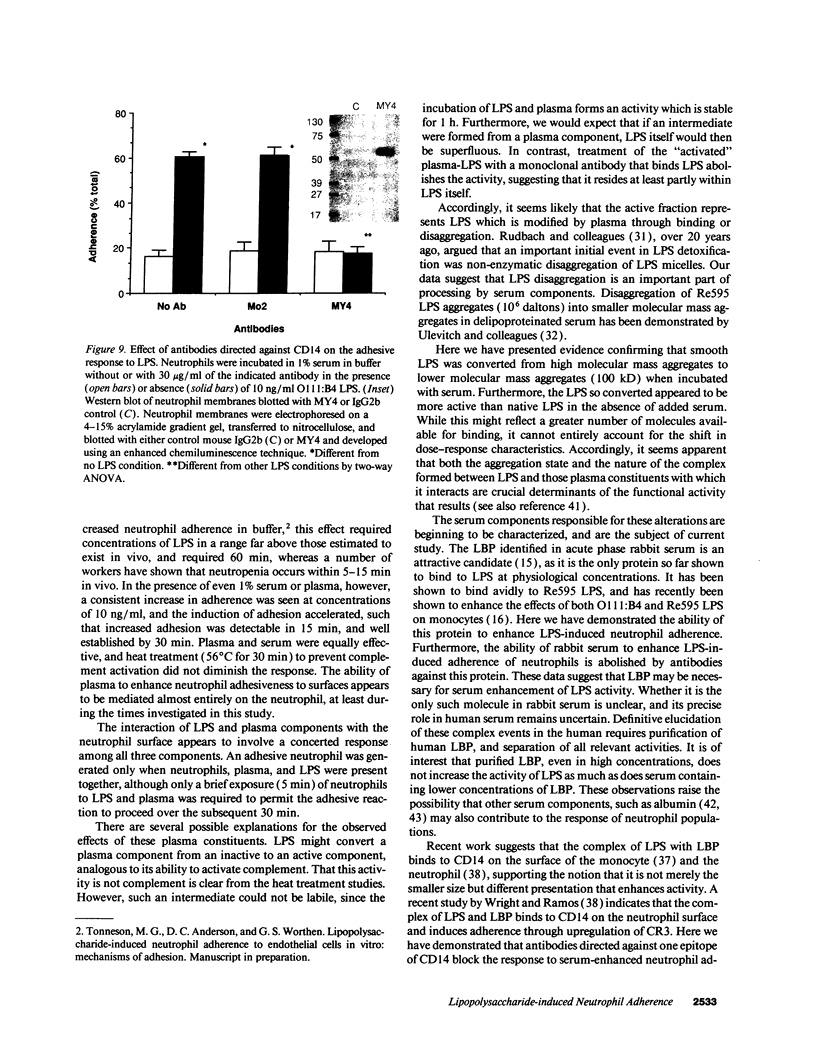
Abstract
Endotoxemia results in neutrophil localization within a number of microcirculatory beds, reflecting in part an adhesive interaction between neutrophils and the vascular endothelial cell. In previous studies, endotoxin or lipopolysaccharide (LPS) treatment of rabbits resulted in neutrophil sequestration at LPS concentrations well below those effective at increasing neutrophil adherence in vitro. We hypothesized that LPS-induced neutrophil adherence involved a plasma component. In the absence of plasma, high concentrations of LPS (10 micrograms/ml) were required to increase human neutrophil adherence to endothelial cells in vitro. With the inclusion of as little as 1% plasma or serum, however, the LPS dose-response curve was markedly shifted, resulting in increments in adherence at 10 ng/ml, and the time course of enhanced adherence was accelerated. Pretreatment studies suggested that the effect of LPS was on the neutrophil rather than the endothelial cell. Immunoprecipitation of 0111:B4 LPS paralleled the loss of functional activity, suggesting that LPS was an integral part of the active complex, rather than altering a plasma component to make it active. The incubation of plasma with LPS decreased the apparent molecular mass of LPS from 500-1,000 kD to approximately 100 kD. The disaggregated 0111:B4 LPS eluted in the range of albumin and was able to increase adherence in the absence of additional plasma. Plasma depleted of lipoproteins or heat treated retained activity, suggesting that the interaction of LPS with HDL or complement did not account for the observed findings. An LPS-binding protein isolated from rabbit serum enhanced the adherence-inducing effects of both 0111:B4 and Re595 LPS. Furthermore, the activity of rabbit serum was abolished after incubation with an antibody directed against this LPS-binding protein (LBP). An antibody directed against CD14, the putative receptor of the LPS-LBP complex, prevented the adhesive response to LPS. These data suggest that LPS is disaggregated by an LBP in serum and plasma to form an active LPS-plasma component complex. This putative complex then interacts with CD14 on the neutrophil so as to induce an adhesive state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide. Requirement for plasma but not for tumor necrosis factor-alpha. J Immunol. 1990 Nov 1;145(9):3017–3025. [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Duncan R. L., Jr, Hoffman J., Tesh V. L., Morrison D. C. Immunologic activity of lipopolysaccharides released from macrophages after the uptake of intact E. coli in vitro. J Immunol. 1986 Apr 15;136(8):2924–2929. [PubMed] [Google Scholar]
- Esko J. D., Matsuoka K. Y. Biosynthesis of phosphatidylcholine from serum phospholipids in Chinese hamster ovary cells deprived of choline. J Biol Chem. 1983 Mar 10;258(5):3051–3057. [PubMed] [Google Scholar]
- FLOREY H. W., GRANT L. H. Leucocyte migration from small blood vessels stimulated with ultraviolet light: an electron-microscope study. J Pathol Bacteriol. 1961 Jul;82:13–17. doi: 10.1002/path.1700820103. [DOI] [PubMed] [Google Scholar]
- Fowler A. A., Hamman R. F., Good J. T., Benson K. N., Baird M., Eberle D. J., Petty T. L., Hyers T. M. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983 May;98(5 Pt 1):593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivuranta-Vaara P., Banda D., Goldstein I. M. Bacterial-lipopolysaccharide-induced release of lactoferrin from human polymorphonuclear leukocytes: role of monocyte-derived tumor necrosis factor alpha. Infect Immun. 1987 Dec;55(12):2956–2961. doi: 10.1128/iai.55.12.2956-2961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Barlow G. H., Sievert H. W., Finley R. A., Yoo A. L. Studies on a lipopolysaccharide from Escherichia coli. Heterogeneity and mechanism of reversible inactivation by sodium deoxycholate. Biochemistry. 1969 Oct;8(10):4063–4067. doi: 10.1021/bi00838a024. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Müller-Berghaus G., Bohn E., Höbel W. Activation of intravascular coagulation by endotoxin: the significance of granulocytes and platelets. Br J Haematol. 1976 Jun;33(2):213–220. doi: 10.1111/j.1365-2141.1976.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Richman A. V., Gerber L. I., Balis J. U. Peritubular capillaries. A major target site of endotoxin-induced vascular injury in the primate kidney. Lab Invest. 1980 Oct;43(4):327–332. [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Cochrane C. G., Henson P. M., Morrison D. C., Doe W. F. Mediation systems in bacterial lipopolysaccharide-induced hypotension and disseminated intravascular coagulation. I. The role of complement. J Exp Med. 1975 Dec 1;142(6):1570–1590. doi: 10.1084/jem.142.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Vosbeck K., Tobias P., Mueller H., Allen R. A., Arfors K. E., Ulevitch R. J., Sklar L. A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990 Feb;47(2):97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Young S. K., Worthen G. S., Haslett C., Tonnesen M. G., Henson P. M. Interaction between chemoattractants and bacterial lipopolysaccharide in the induction and enhancement of neutrophil adhesion. Am J Respir Cell Mol Biol. 1990 Jun;2(6):523–532. doi: 10.1165/ajrcmb/2.6.523. [DOI] [PubMed] [Google Scholar]