| Title: | Compounds Useful as Immunomodulators | ||
| Patent Application Number: | WO 2015/034820 Al | Publication date: | 12 March 2015 |
| Priority Application: | US 61/873,398 | Priority date: | 4 September 2013 |
| Inventors: | Chupak, L. S.; Zheng, X. | ||
| Assignee Company: | Bristol-Myers Squibb Company; Route 206 and Province Line Road, Princeton, NJ 08543, USA | ||
| Disease Area: | Cancer and infectious diseases such as hepatitis C | Biological Target: | PD-1/PD-L1 pathway |
| Summary: | The invention in this patent application relates to compounds represented generally by formula (I), which possess activities as inhibitors of the PD-1/PD-L1 interactions and therefore may potentially be useful in the treatment of cancer as well as infectious diseases such as hepatitis C. | ||
| T-cells or lymphocytes are white blood cells that are essential for the immune system. They are capable of searching for and destroying infected and/or cancerous cells. Programmed death protein 1 (PD-1), also known as cluster of differentiation 279 (CD279), is a cell surface receptor on the T cells. The binding of PD-1 with either one of its two known ligands, programmed death-ligands 1 and 2 (PD-Ll or PD-L2), has been shown to suppress T cell receptor activating signals. The PD-l/PD-L1 pathway down regulates the immune responses during resolution of an infection or a tumor, or during the development of self-tolerance. | |||
| Studies have shown that blocking the PD-1/PD-Ll interactions using antibodies to the PD-Ll protein restores and augments T cell activation in many systems. A recent study has shown that therapy with a monoclonal antibody to PD-Ll benefited patients with advanced cancer. Blocking the PD-1/PD-Ll pathway by monoclonal antibodies enhanced the immune response and resulted in tumor rejection or control of infection in preclinical animal models. It can also restore in vitro antigen-specific functionality to T cells from HIV, HCV, or HBV patients. Other reports show that blocking the PD-1/PD-L1 interaction enhances T cell activity in chronic infection systems and may augment therapeutic immune response to a number of histologically distinct tumors. It also enhances the responses to vaccination, including therapeutic vaccination in chronic infections. | |||
| The term “T cell exhaustion” describes the conditions of the T cells resulting from chronic antigen stimulation that occurs during chronic infections and tumor disease. These cells are characterized by elevated levels of PD-1 and dysfunctional activities toward chronic antigen. Targeting PD-L1 protein to inhibit the PD-1/PD-L1 pathway has been shown to restore antigen-specific T cell immune functions in vitro and in vivo, including enhanced responses to vaccination in the setting of tumor or chronic infection. | |||
| The inhibition of the interaction of PD-Ll with PD-1 is thus a viable and promising therapeutic target for the treatment of cancer and/or chronic infections. The invention in this patent application presents compounds with activities as inhibitors of the PD-1/PD-Ll protein/protein interactions. These compounds may potentially be useful therapy to enhance immunity in patients with cancer or chronic infections. | |||
| Important Compound Classes: | 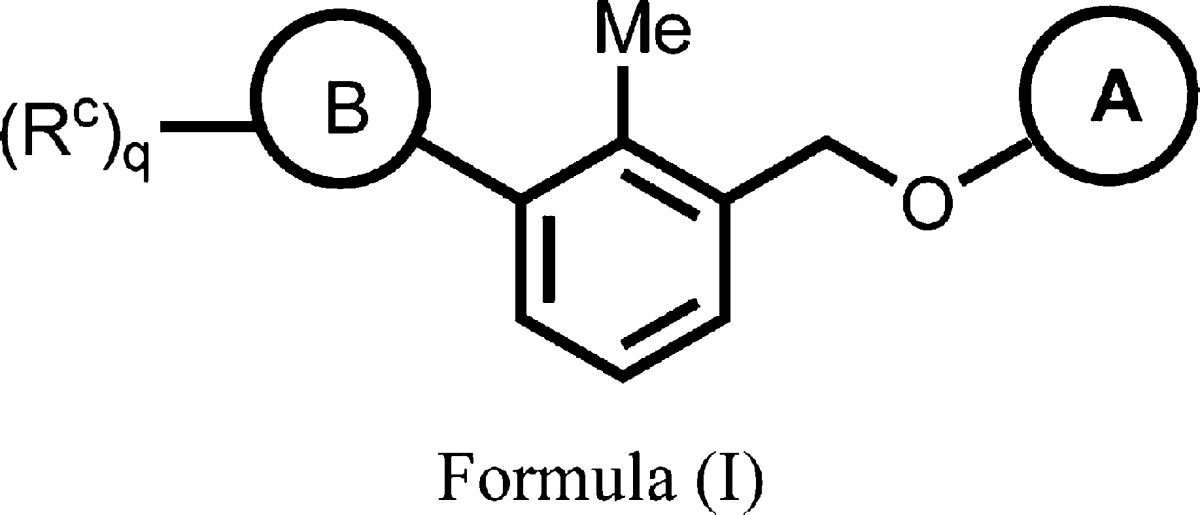 |
||
| Key Structures: | The inventors listed the structures of 297 examples of formula (I) including the following six representative examples: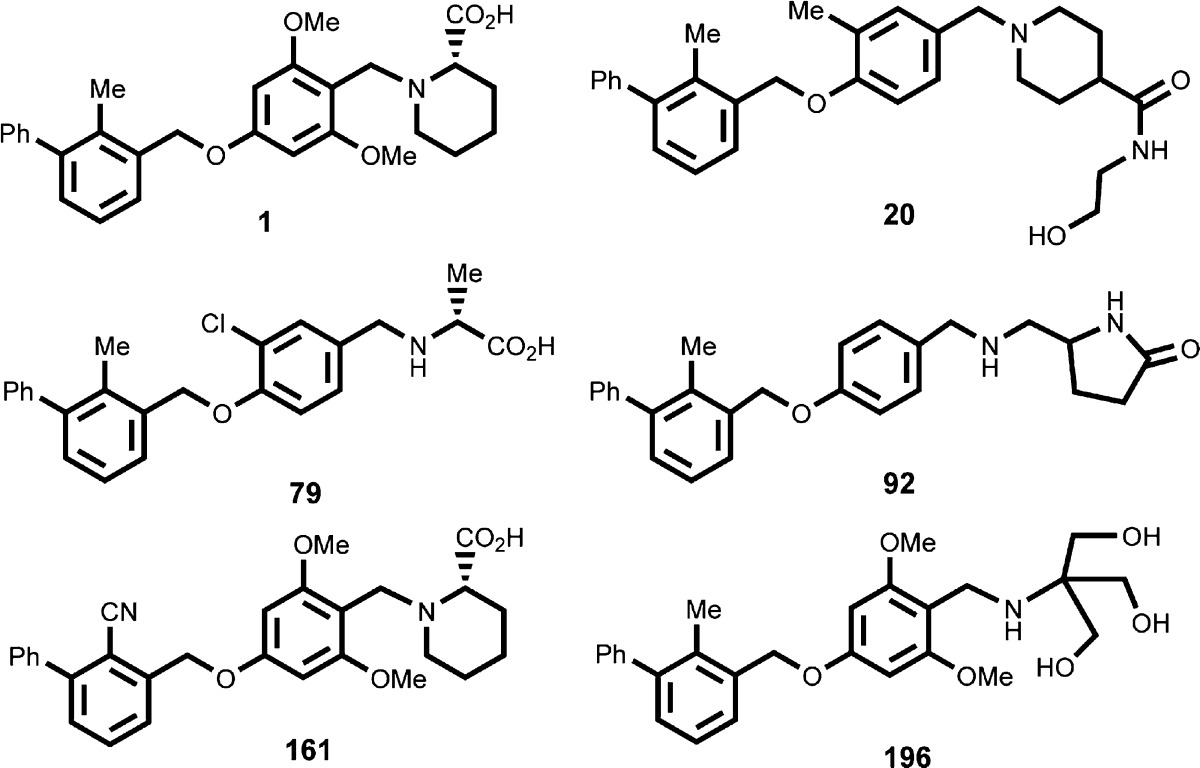
|
||
| Biological Assay: |
|
||
| Biological Data: | The inventors listed the IC50 data from the HTRF binding assay for the 297 examples of formula (I). The following table contains the assay data for the above six representative examples: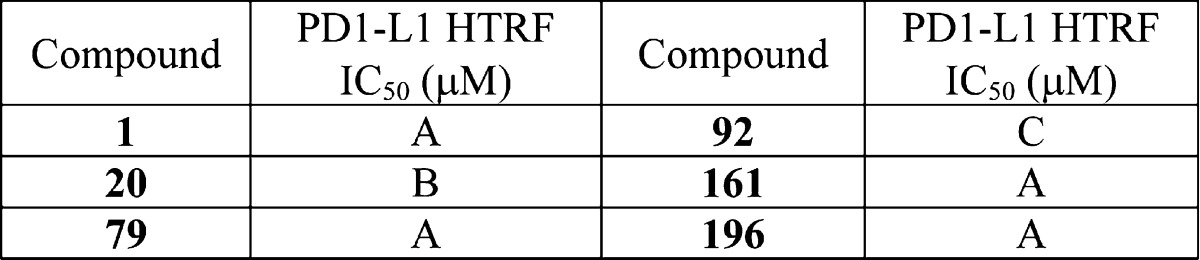
|
||
| Codes for IC50 values: A = 0.006–0.10 μM; B = 0.11–1.00 μM; C = 1.01–10 μM | |||
| Recent Review Articles: | 1. Muenst S.; Soysal S. D.; Tzankov A.; Hoeller S.. Expert Opin. Ther. Targets 2015, 19 ( (2), ), 201–211. | ||
| 2. Ohaegbulam K. C.; Assal A.; Lazar-Molnar E.; Yao Y.; Zang X.. Trends Mol. Med. 2015, 21 ( (1), ), 24–33. | |||
| 3. Saresella M.; Rainone V.; Al-Daghri N. M.; Clerici M.; Trabattoni D.. Curr. Mol. Med. 2012, 12 ( (3), ), 259–267. | |||
| 4. Larrubia J. R.; Benito-Martinez S.; Miquel J.; Calvino M.; Sanz-de-Villalobos E.; Parra-Cid T.. World J. Gastroenterol. 2009, 15 ( (41), ), 5129–5140. | |||

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.