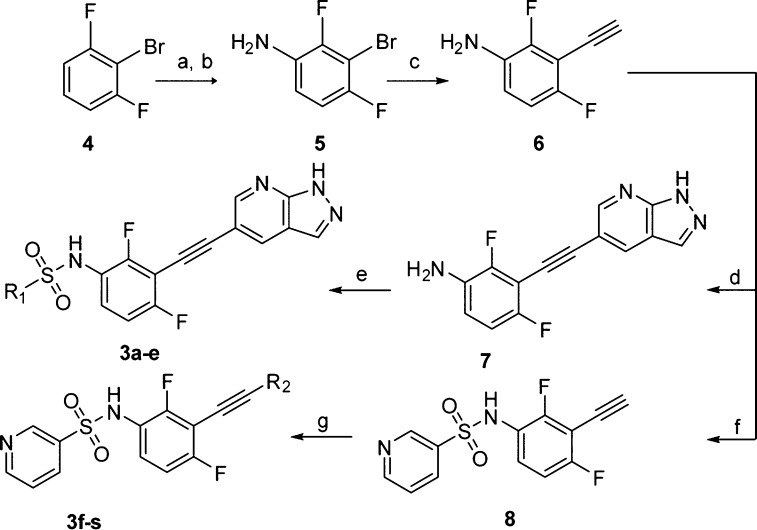
Scheme 1. Synthesis of Compounds 3a–3s.
Reagents and conditions: (a) con. H2SO4, KNO3, rt, 2.0 h, 91%; (b) SnCl2, con. HCl, EtOH, reflux, 93%; (c) (i) Pd(dba)2, CuI, ethynyltrimethylsilane, t-(Bu)3P, K2CO3, dry THF, 120 °C, 48 h; (ii) n-(Bu)4NF, THF, rt, 1 h, 46%; (d) 6-bromo-3H-pyrazolo[3,4-b]pyridine, Pd(dba)2, CuI, t-(Bu)3P, K2CO3, dry THF, 120 °C, 24 h, 63%; (e) R1Cl, pyridine, dry DCM, rt, overnight, 39–90%; (f) pyridine-3-sulfonyl chloride, pyridine, dry DCM, rt, overnight, 93%; (g) R2Br, Pd(dba)2, CuI, t-(Bu)3P, K2CO3, dry THF, 120 °C, 24 h, 47–70%.