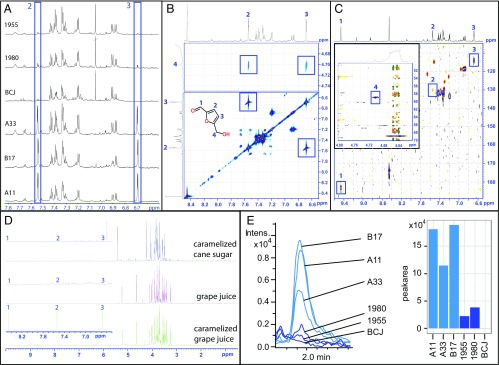
Fig. 2.
Characterization of 5-hydroxymethylfurfural (HMF) in the Baltic Sea champagne samples. (A) 1H NMR spectra (spectral region 7.8–6.6 ppm) illustrating the presence or absence of HMF in the six different champagne samples (blue boxes). (B) Molecular structure of HMF and carbon annotation. (B and C) Identification of HMF via (B) 2D-1H-1H DIPSI and (C) 2D-1H-13C-NMR (blue boxes) (D) Determination of the possible origin of HMF in the Baltic champagne samples. Absence of HMF (with focus on carbons 1, 2, and 3) in caramelized cane sugar and unheated grape juice vs. presence of HMF in heat-treated grape juice. (E) Quantification of HMF by UPLC-MS. High-resolution–extracted ion chromatogram of mass 125.024418 ± 0. 005 Da corresponding to [M-H]− ion of hydroxymethylfurfural in different champagne samples analyzed under reversed-phase conditions and peak area of the chromatographic peak of hydroxymethylfurfural.