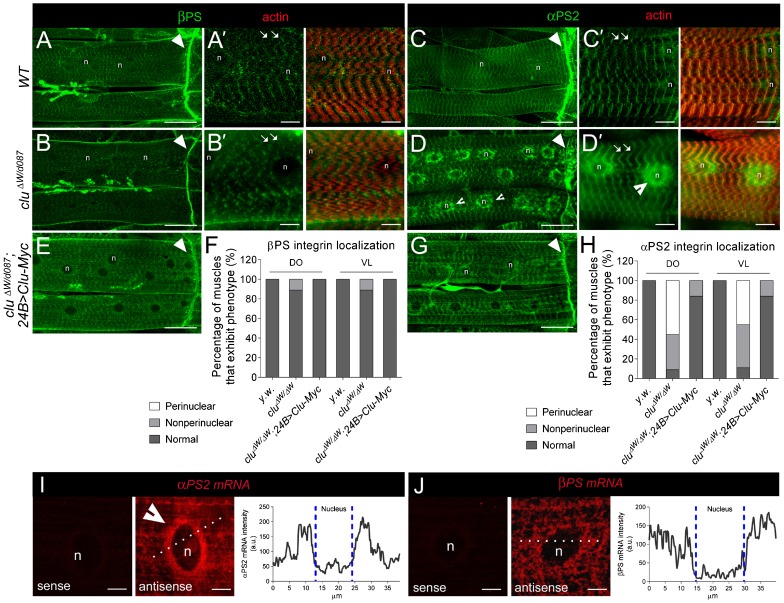
Fig. 1. αPS2 accumulates within contractile muscles upon loss of Clu.
(A–E,G) Immunolocalization of integrin proteins (green) and F-actin (red; phalloidin) in muscles of filleted L3 individuals (n = nucleus). (A–B′) In both WT and clu−/− muscles, βPS integrin is found at MASs (arrowheads in A,B) and costamere structures that encircle the sarcolemma along the length of the muscle (arrows in A′,B′). (C,C′) αPS2 integrin also accumulates at the ends of WT muscles (arrowhead in C) and at costameres (arrows in C′). (D,D′) In clu mutants, the αPS2 subunit accumulates around the periphery of the nucleus, as indicated by the indented arrowheads. (E,G) The reintroduction of full-length clu cDNA into clu mutant muscle tissue has no effect on βPS integrin distribution (E) and restores the accumulation of αPS2 to its normal location within the cell (G). (F,H) Quantification of βPS and αPS2 integrin distribution in the dorsal oblique (DO; 16<n<36) and ventral longitudinal (VL; 16<n<36) L3 muscles of indicated the genotypes. (I,J) Fluorescent in situ hybridizations (FISH) in L3 muscle tissue. αPS2 mRNA accumulates around the nuclei (I, middle panel; n = 42), while βPS mRNA appears evenly distributed throughout the muscle cell (J; n = 23). The sense probes for both mRNAs reveal little background signal (left panels). Quantitation of fluorescence intensity (dotted line) shows that the perinuclear signal of αPS2 mRNA is higher than that of βPS2 mRNA. Scale bars, 50 µm (A–E,G), 10 µm (A′–D′), 5 µm (I,J).