Fig. 2.
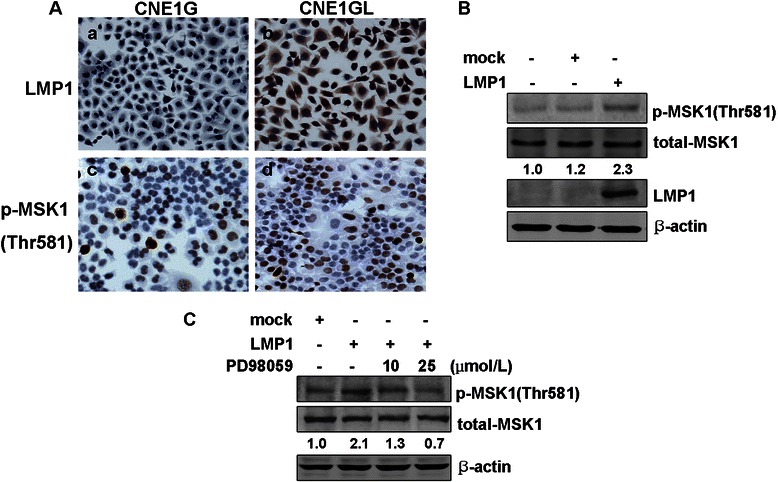
LMP1 induces phosphorylation of MSK1 at Thr581 in CNE1 cells. (a) CNE1G and CNE1GL cells were starved for 36 h by incubating in serum-deprived 1640 and subjected to immunocytochemical staining. No expression and positive staining of LMP1 (a, b) and different expression of phosphorylated MSK1 at Thr581 (c, d) in CNE1G and CNE1GL cells (magnification, ×200). (b) CNE1 cells were transfected with pcDNA3.0 or pcDNA3.0-LMP1. After 24 h of transfection, cells were starved for another 24 h by incubating in serum-deprived 1640 and then harvested. The expressions of LMP1 and phosphorylated MSK1 at Thr581 were detected by Western blotting. (c) CNE1 cells were transfected with pcDNA3.0 or pcDNA3.0-LMP1 vector. PD98059 was added to the culture medium at the concentration indicated every 12 h after transfection. The expression of phosphorylated MSK1 was detected by Western blotting. β-actin was used as loading control. Corresponding signaling intensities of phosphorylated MSK1 were densitometrically determined and normalized to total MSK1 in each lane and is given below in each data
