Abstract
Background
Transversus abdominis plane (TAP) block has been shown to ameliorate postoperative pain after abdominal surgery. Postoperative pain-associated respiratory compromise has been the subject of several studies. Herein, we evaluate the effect of oblique subcostal TAP (OSTAP) block on postoperative pain and respiratory functions during the first 24 postoperative hours.
Material/Methods
In this double-blind, randomized study, 76 patients undergoing laparoscopic cholecystectomy were assigned to either the OSTAP group (n=38) or control group (n=38). Bilateral ultrasound-guided OSTAP blocks were performed with 20 ml 0.25% bupivacaine after induction of general anesthesia. Both the OSTAP and control groups were treated with paracetamol, tenoxicam, and tramadol as required for postoperative analgesia. Visual Analog Scale (VAS) pain scores (while moving and at rest), forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), peak expiratory flow rate (PEFR), arterial blood gas variables, and opioid consumption were assessed during first 24 h.
Results
VAS pain scores at rest and while moving were significantly lower in the OSTAP group on arrival to PACU and at 2 h postoperatively. The total postoperative tramadol requirement was significantly reduced at 0–2 h and 2–24 h in the OSTAP group. Postoperative deterioration in FEV1 and FVC was significantly less in the OSTAP group when compared to the control group (P<0.01 and P<0.05, respectively). There were no between-group differences in arterial blood gas variables.
Conclusions
After laparoscopic cholecystectomy, OSTAP block can provide significant improvement in respiratory function and better pain relief with lower opioid requirement.
Keywords: Anesthesia, Conduction; Cholecystectomy, Laparoscopic; Pain, Postoperative; Respiratory Function Tests
Background
Postoperative pain is among the primary causes of reduction in respiratory function after upper abdominal surgery [1]. Epidural analgesia is preferred for postoperative pain control and restoration of respiratory function, but associated complications and contraindications may limit its use in outpatient surgery [2–5]. As a newer technique, transversus abdominis plane (TAP) block is easier and perhaps safer as a part of multi-modal analgesia. Theoretically, TAP block may replace the need for epidural analgesia after abdominal operations. The oblique subcostal approach of the TAP (OSTAP) block, which was described by Hebbard et al. [6], has been reported to provide analgesia to the entire anterior abdomen. Its potential to reduce postoperative pain among patients undergoing abdominal surgery has been demonstrated in several studies [7,8]. To the best of our knowledge, there are no data on its positive effects on lung function. Therefore, the aim of this study was to evaluate the effects of OSTAP block in control of postoperative pain and respiratory function after outpatient laparoscopic cholecystectomy.
Material and Methods
The study was performed at Konya Training and Research Hospital, Konya, Turkey. All study documents and procedures were approved by the institutional review board at the University of Selçuk, Konya, Turkey (Chairperson Dr. Gergerlioglu) on May 22, 2014 (protocol no. B.30.2.SEL.0.28.00.00/130). All subjects provided written informed consent prior to initiation of study procedures. The study protocol conforms to the ethics guidelines of the 2013 Declaration of Helsinki [9].
Participants
Adult patients with ASA physical status I–II that were scheduled for laparoscopic cholecystectomy as outpatient surgery were enrolled. Patients were randomly assigned either to OSTAP block with 0.25% bupivacaine (OSTAP study group) or to the control group, with an allocation ratio of 1:1. Exclusion criteria were: age <18 and >65, inability to speak Turkish, pregnancy, alcohol or drug abuse, chronic opioid intake, consumption of any pain killers within the 24 h before the operation, infection at injection site, respiratory tract infection within the last 2 weeks, smoker or history of smoking, allergy to local anesthetics, current or past lung and allergic diseases including asthma, rhinitis or atopic dermatitis, cardiac disease associated with dyspnea - New York Heart Association class >II, severe psychiatric disorders and history of abdominal surgery or trauma, or conversion of laparoscopic to open surgery.
Randomization and blinding
The study was randomized and double-blind. The randomization was done using the permutated block randomization method. The randomization scheme was generated by using the website Randomization.com (http://www.randomization.com). Randomization was performed by AB in 21 blocks with 4 subjects per block. Sequentially numbered, opaque, sealed envelopes were prepared and provided to investigators BB and BK, who administered the OSTAP and anesthesia. OSTAP block was performed after induction of anesthesia. In the OSTAP and control groups, block sites were covered with dressings, and patients were blinded to treatment allocation. Investigator EK, who was involved in recording postoperative parameters of the patients, was blinded to study groups.
Preoperative management
On the day of the operation, the visual analog scale (VAS), graduated from 0 (no pain) to 10 (the worst imaginable pain), was explained to patients. Additionally, patients were informed about the 4-point scale for postoperative nausea (0=none; 1=mild; 2=moderate; 3=severe). All patients were provided instructions about the use of a hand-held spirometer. A radial artery catheter was inserted under local anesthesia for arterial blood gas analysis in all patients.
Anesthesia
Patients were premedicated with 10 mg diazepam PO after placement of radial artery catheter and spirometric measurements, which was done approximately 1 h before induction. All patients received standardized anesthesia protocol, which included standard monitoring (ECG, noninvasive blood pressure, pulse oximetry, CO2 analyzer, and TOF monitoring) and continuous intraoperative infusion of 5 ml/kg/h 0.9% sodium chloride solution.
Anesthesia was induced with propofol 1–1.5 mg/kg and fentanyl 2 μg/kg. Tracheal intubation was facilitated by rocuronium 0.6 mg/kg. Anesthesia was maintained with sevoflurane at 1 minimum alveolar concentration corrected for age in an air: oxygen mixture with an FiO2 of 0.4 and 0.1 μg/kg/min remifentanil infusion. Neuromuscular blockade was monitored by train of four (TOF) stimulation. Repeated doses of rocuronium 0.1 mg/kg were given to maintain muscular relaxation until extraction of laparoscopic ports. An Avance S/5® (Datex Ohmeda) ventilator was used for ventilation with pressure control mode. End-tidal CO2 was maintained between 30–35 mmHg, and a PEEP of 5 cm H20 was applied to all patients. Remifentanil and sevoflurane administration were stopped after extraction of laparoscopic ports. All patients received meperidine 0.5 mg/kg IV before remifentanil cessation. Ephedrine 5 mg IV was administered for 20% reduction in initial mean arterial blood pressure (MAP) or <60 mmHg, and additional doses of ephedrine were permitted after 2 min. Bolus doses of fentanyl (1 μg/kg) were given if the MAP or heart rate (HR) of the patient increased 20% of initial value. Neostigmine 0.05 mg/kg and atropine 0.01 mg/kg were given intravenously as needed to antagonize neuromuscular block. Then, patients were extubated when fully awake with TOF ratio ≥0.90. Following emergence from anesthesia, patients were placed in a 30-degrees upright position and given 2 l/min oxygen via nonrebreathing facemask with reservoir bag.
Oblique subcostal transversus abdominis plane block
After induction of general anesthesia, ultrasound-guided bilateral oblique subcostal transversus abdominis plane (OSTAP) block were performed with Mindray M5® ultrasound system and linear ultrasound transducer (8–12 Hz) 15 min before the surgical incision by 1 of 2 investigators (BB or BK).The transversus abdominis and rectus abdominis muscles were identified near the costal margin and xiphoid process. A Pajunk® 22 gauge 80-mm needle (SonoPlex Stim, USA) was introduced through the rectus muscle 2 to 3 cm medial to the probe. The probe was positioned to image the needle in plane. After the tip of the needle was visualized in between the rectus and transversus abdominis muscle, 20 cc of 0.25% bupivacaine were injected following verification of needle position with 2 ml saline. Likewise, OSTAP block was performed on the contralateral side.
Surgery
Laparoscopic cholecystectomy was achieved using 4 ports; two 5-mm and two 10-mm ports that were placed supra- and infra-umbilically. Pneumoperitoneum was produced using Veress needle technique. The gallbladder was retracted through the port site cranial to the umbilicus. All port sites were closed using resorbable sutures.
Postoperative analgesia and antiemetic use
A standardized postoperative analgesic regimen was used consisting of paracetamol 500 mg PO every 6 h initiated 30 min before surgery and tenoxicam 20 mg IV after induction of anesthesia. Ondansetron 4 mg IV was administered after laparoscopic ports were extracted. For the first 2 h in the postoperative care unit, tramadol 50 mg IV was given by a nurse on request of patients, with minimum 20 min between doses. After 2 h, tramadol 50 mg IV was given based on initial postoperative analgesic requirement protocol with minimum 30 min following the last tramadol dose. The maximum tramadol dose was limited to 500 mg at 2–24 h postoperatively.
In case of nausea, patients were asked about its severity as preoperatively emphasized; given metoclopramide 5 mg IV to a patient whose nausea score was ≥2. Maximum metoclopramide dose was 20 mg for the first postoperative 24 hours.
Spirometric measurements
All tests were performed at bedside with the patients in a sitting or semi-recumbent position. At least 3 acceptable measurements were done to meet the European Respiratory Society criteria for reproducibility [10]. Forced vital capacity (FVC), forced expiratory volume in first second (FEV1), and peak expiratory flow rate (PEFR) measured by the use of hand-held spirometer (One-flow®, Clement Clarke, U.K.) by the same blinded investigator (EK). At each assessment, the largest values of FEV1, FVC, FEV1/FVC, PEFR were recorded.
Arterial blood-gas analysis
Before each spirometric measurement, arterial blood sample was drawn for pH, pCO2, pO2, HCO3, and oxygen saturation (SO2). Samples were obtained with 2-ml syringes containing heparin and analyzed with the RapidLab® 1265 blood gas analyzer (Siemens, Germany). After the operation, each patient received 2 l/min oxygen via nonrebreathing facemask for the first postoperative hour. Oxygen administration was only increased if necessary to keep SO2 >92%; During the second h, the oxygen were administered to patients whose SO2 were below 92%; and these patients were disconnected from the oxygen mask for 10 min or to the time when SO2 was below the 88% before arterial blood gas sampling was drawn.
Data collection
Before the operation, age, weight, height, sex, ASA classification, arterial blood gas analysis, and spirometric measurements of the patients were assessed. Total dose of intraoperative fentanyl, ephedrine consumption, and surgical duration were recorded. Pain scores at rest and while moving (cough and turn around) were collected on arrival to the postanesthesia care unit (PACU), 2 h (before discharge from PACU) and 24 h after the operation.
Sedation score (4-point score; 0 – does not open eyes to verbal commands; 1 – sleeping, easy to arouse verbally; 2 – drowsy; 3 – alert), nausea score (4-point score; 0 – none; 1 – mild; 2 – moderate; 3 – severe), spirometric and arterial blood gas analysis in the OSTAP and control groups were recorded at 2 and 24 h postoperatively.
Opioid and antiemetic consumptions were recorded as on-request tramadol IV and metoclopramide IV for the first 2 and 2–24 postoperative hours.
Finally, the number of episodes of vomiting (>10 mL) and number of patients receiving metoclopramide were recorded.
Outcomes
The primary outcome variable was VAS pain scores during movement on arrival at the PACU. Secondary outcomes included pain scores while moving, at the other timepoints pain scores were assessed at rest and on arrival to the PACU, at 2 and 24 h postoperatively, and postoperative opioid consumption at the first 2 and 2–24 h after the operation. Other secondary outcome variables were respiratory function assessed by spirometric parameters (FEV1, FVC, FEV1/FVC, and PEFR) and pH, pO2, pCO2, HCO3, and SO2 in arterial blood gas analysis at postoperative 2 and 24 h, postoperative nausea and sedation scores, and number of vomiting incidents.
Sample size
The expected VAS score in the placebo group was estimated from the TAP block study of Petersen et al. [11]. We estimated that with a sample size of 76 patients, the study would have 90% power to show a 40% reduction in the rate of the primary outcome, with a 2-sided type I error rate of 5%. To calculate the sample size, we used the power and sample size module of Minitab® 16 Statistical Software ([Computer software]. State College, PA: Minitab, Inc. [www.minitab.com]).
Statistical analysis
Data from all patients were analyzed according to the group to which they were randomly assigned on a per protocol basis.
Normal distributions of the variables were assessed via Kolmogorov-Smirnov test, Q-Q plots, and histograms. For continuous variables, mean ± standard deviation and for ordinal and categorical variables median and ranges were reported. Baseline characteristics of the study and control groups were compared using the t-test for normally distributed continuous variables and Mann-Whitney U test for continuous variables without normal distribution and ordinal variables. Categorical variables were compared using the chi-square test. All statistical tests that were used to compare 2 groups were 2-sided with a significance level of 0.05; analyses were exploratory, and did not adjust for multiple testing. Data analysis and management were performed using IBM SPSS® 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp).
Results
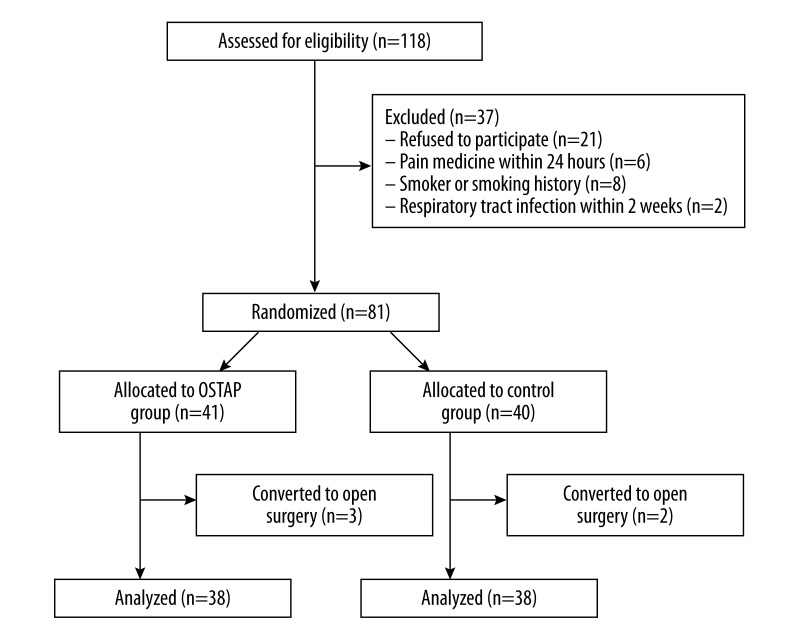
Eighty-one patients were randomly allocated between May 2014 and October 2014 (Figure 1). Five patients were excluded (2 patients in the control group and 3 patients in OSTAP group were converted to open surgery). Seventy-six patients were included in the statistical analysis (Figure 1).
Figure 1.
Flow diagram. (OSTAP: oblique subcostal transversus abdominis plane).
Patients’ characteristics and perioperative data are provided in the Table 1, and were similar in both groups. Intraoperative fentanyl and ephedrine consumption did not show differences between groups. None of the patients suffered from any serious complications related to OSTAP block.
Table 1.
Patient characteristics and perioperative data.
| Control group (n=38) | OSTAP group (n=38) | P | |
|---|---|---|---|
| Age (years) | 44.89±14.2 | 43.2±12.2 | 0.591 |
|
| |||
| Gender | |||
| Female | 36 (47.4%) | 29 (38.2%) | 0.047 |
| Male | 2 (2.6%) | 9 (11.8%) | |
|
| |||
| Weight (kg) | 77.6±10.3 | 77.3±11.4 | 0.903 |
|
| |||
| Length (cm) | 161.3±7.8 | 162.1±9.3 | 0.689 |
|
| |||
| BMI (kg/m2) | 29.9±3.9 | 29.5±4.5 | 0.713 |
|
| |||
| ASA PS | 1 (1) | 1 (1) | 1.0 |
|
| |||
| Operation time (min) | 40.9±12.2 | 40.8±20.2 | 0.462 |
|
| |||
| Anesthesia time (min) | 57.1 ±13.4 | 55.9±21.6 | 0.42 |
|
| |||
| Fentanyl consumption (μg) | 19.4±47.6 | 9.1±27.4 | 0.437 |
|
| |||
| Ephedrine consumption (mg) | 0.26±1.6 | 0.39±1.8 | 0.48 |
Data were presented as means ±SD, median (range), counts (percentage). OSTAP – oblique subcostal transversus abdominis plane; BMI – body mass index; ASA PS – American Society of Anesthesiologist Physical Status.
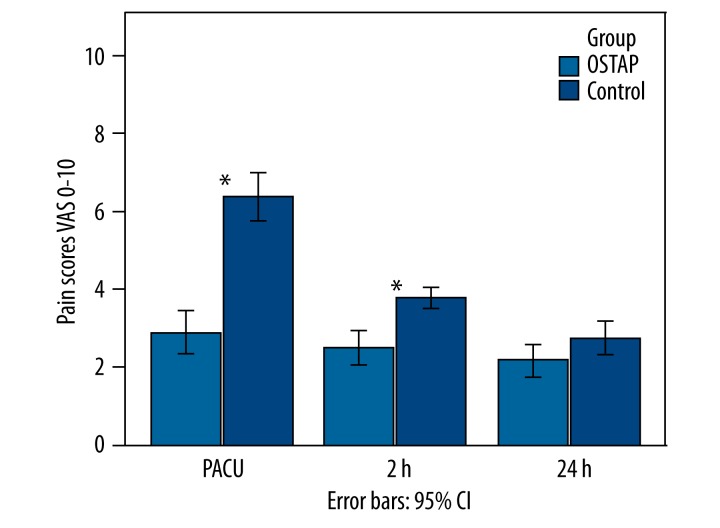
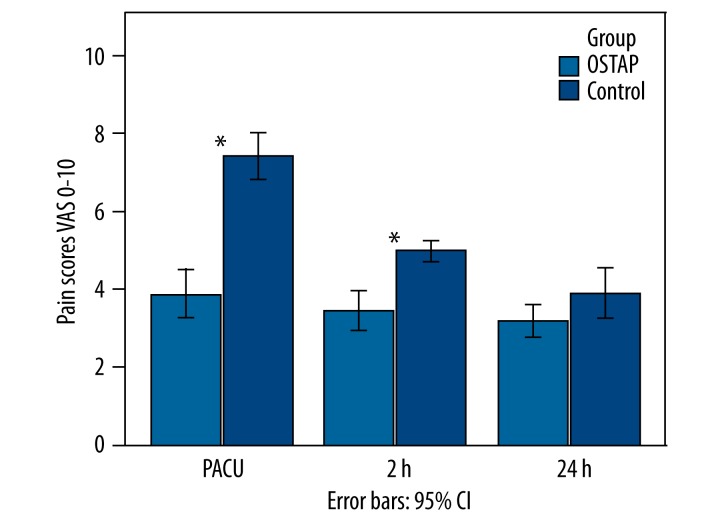
Patients who received OSTAP block had lower VAS pain scores at rest than the patients in the control group at all timepoints except 24 h (Figure 2). Comparisons of VAS scores while moving demonstrated significantly higher values in the control group at all timepoints except 24 h (Figure 3).
Figure 2.
VAS pain scores at rest. Data were presented as mean. P-value comparison between the OSTAP and control groups – * P<0.01. (OSTAP: oblique subcostal transversus abdominis plane; PACU: Postanesthesia Care Unit).
Figure 3.
VAS pain scores on movement. Data were presented as mean. P-value comparison between the OSTAP and control groups – * P<0.01. (OSTAP: oblique subcostal transversus abdominis plane; PACU: Postanesthesia Care Unit).
Postoperative 0–2 h and 2–24 h tramadol consumption was significantly lower in the OSTAP group (Table 2). Levels of sedation, nausea, number of patients vomiting, number of vomiting episodes, and consumption of metoclopramide were not significantly different between the groups (Table 3).
Table 2.
0–2, 2–24 hours analgesic consumption and 0–24 hours metoclopramide consumption.
| Control group | OSTAP group | P | |
|---|---|---|---|
| Tramadol consumption 0–2 h (mg) | 80.3±50.1 | 31.6±31.7 | 0.001 |
| Tramadol consumption 2–24 h (mg) | 267.1±108.6 | 126.3±54.2 | 0.001 |
| Metoclopramide consumption 0–24 h (mg) | 3.3±5.5 | 2.3±3.7 | 0.719 |
Data were presented as means ±SD. OSTAP – oblique subcostal transversus abdominis plane.
Table 3.
Postoperative nausea, vomiting and sedation scores.
| Control group | OSTAP group | P | |
|---|---|---|---|
| Nausea score | |||
| 2 h | 0 (3) | 0 (3) | 0.389 |
| 24 h | 0 (2) | 0 (1) | 0.325 |
|
| |||
| No of patients vomiting/24 h | 10 | 8 | 0.788 |
|
| |||
| No of vomiting episodes/24 h | 0 (3) | 0 (2) | 0.451 |
|
| |||
| No of patients requiring metoclopramide/24 h | 12 | 12 | 1.0 |
|
| |||
| Sedation score | |||
| 2 h | 3 (1) | 3 (1) | 0.576 |
| 24 h | 3 (0) | 3 (0) | 1.0 |
Data were presented as median (range) or counts. OSTAP – oblique subcostal transversus abdominis plane.
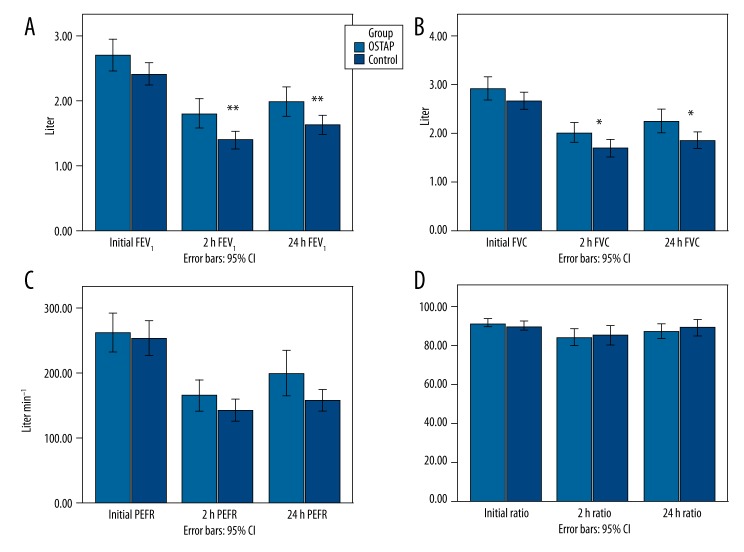
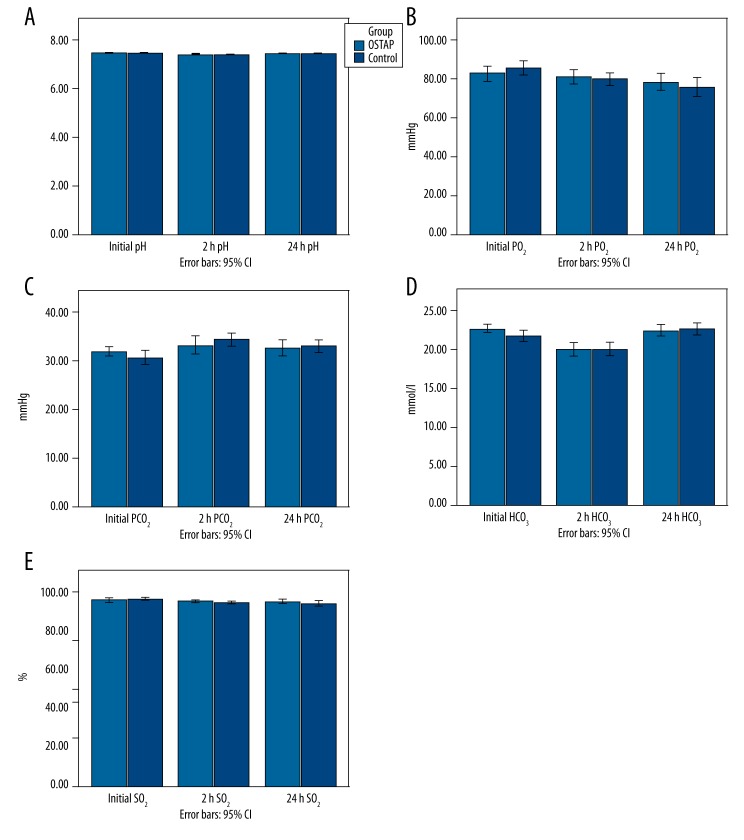
Changes in spirometric variables at all the timepoints are shown in Figure 4. Patients in the OSTAP group had significantly higher postoperative FEV1 values at 2 (p=0.002) and 24 h (p=0.008) when compared with those in the control group. Similarly, the OSTAP group had better FVC values at 2 (p=0.029) and 24 h (p=0.019). FEV1/FVC and PEFR values were similar between groups. Arterial blood gas parameters (pH, pO2, pCO2, HCO3, SO2) were similar between groups (Figure 5).
Figure 4.
Time-course of respiratory function FEV1 (A), FVC (B), PEFR (C), FEV1/FVC ratio (D) ([FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PEFR: peak expiratory flow rate; Ratio: FEV1/FVC], [Initial: preoperative values; 2 h: postoperative 2 h; 24 h: postoperative 24 h; OSTAP: oblique subcostal transversus abdominis plane]). P-value comparison between the OSTAP and control groups – * P<0.05, ** P<0.01.
Figure 5.
Time course of arterial blood gas variables. Data are presented as mean ([pH: acidity; pO2: partial oxygen pressure; pCO2: partial carbon dioxide pressure; HCO3: bicarbonate concentration; SO2: oxygen saturation], [Initial: preoperative values; 2 h: postoperative 2 h; 24 h: postoperative 24 h; OSTAP: oblique subcostal transversus abdominis plane]).
Discussion
The major findings of our study were better preservation of lung volume, lower pain scores at rest and while moving during the early postoperative period in patients undergoing outpatient laparoscopic cholecystectomy with the application of single-injection bilateral OSTAP block as a part of multimodal analgesia. Furthermore, reduced tramadol consumption was observed in patients treated with OSTAP block during the first 2 and 2–24 h postoperative.
Wu et al. [12] reported that single-injection subcostal TAP block provides effective postoperative analgesia at rest and while moving compared to intravenous opioid analgesia during upper abdominal surgery. In the same study, continuous thoracic epidural analgesia was shown to provide more effective analgesia than single-injection subcostal TAP block [12]. Very recently, in patients undergoing laparoscopic cholecystectomy, OSTAP block was reported to produce better analgesia than classic TAP block or intravenous opioid analgesia during the postoperative 24-h period [8]. In contrast, Chen et al. [13] observed no difference in analgesic efficacy between OSTAP block and intravenous morphine during the postoperative period. In the current study, we observed lower pain scores in the PACU (Figures 2 and 3), and reduced tramadol consumptions in the first 2 and 2–24 postoperative h in the OSTAP group.
Reduced respiratory function after upper abdominal surgery may last up to 10 days and is characterized by restrictive pattern. Diaphragmatic dysfunction, pain,and muscular disruption are listed as the main causes of this deterioration [14,15]. Accordingly, patients have limited capacity to use their abdominal muscles for prevention of pulmonary stagnation by coughing and deep breathing.
Several studies have evaluated postoperative pain-related respiratory compromise and effects of different analgesic modalities on lung functions. A meta-analysis performed by Ballantyne et al. [16] showed that wound infiltration of local anesthetics did not improve respiratory functions. Only 1 of the trials reviewed by the authors studied the effect of facial infiltration of local anesthetics, which failed to demonstrate any benefit to postoperative respiratory functions [17]. Among the several analgesic methods used in upper abdominal surgery, only epidural analgesia was shown to provide beneficial effects in the recovery of respiratory functions [18,19].
Spirometric measurements and arterial blood gas analysis were the most commonly used parameters to analyze overall respiratory function after laparoscopic cholecystectomy. Rademaker et al. [20] found a reduction of approximately 30–45% in FEV1 and FVC at 2 h and 24 h after laparoscopic cholecystectomy. Our results were comparable with their findings. In contrast, other studies reported 18–20% reduction in FEV1 and FVC at 24 h after laparoscopic cholecystectomy [5,21]. Epidural analgesia was reported to result in 15% improvement in respiratory volumes compared with systemic analgesia following open upper-abdominal surgery [19]. Previous data regarding the effect of epidural analgesia after laparoscopic abdominal surgery were few and did not include more than 10 patients in each group. Only 1 study, which utilized epidural analgesia, reported that respiratory function was slightly improved; however, the authors regarded the difference as clinically insignificant [20]. In this context, epidural analgesia was not considered as a postoperative analgesic regimen in studies on its effect on respiratory functions of patients undergoing outpatient surgery. Probably its potential risks outweighed the benefits, which include pain control and improved respiratory function during outpatient surgeries [3,22,23].
Breathing is a complex physiologic process consisting of rhythmic changes in lung volume involving several muscles, which are controlled by the medullary respiratory neurons [24,25]. In general, abdominal muscles are expiratory. The rectus abdominis, external oblique, internal oblique, and transversus abdominis muscles take part in varying degrees with respiratory work and are mainly active in forced expiration [26]. Previous studies have examined separate contributions of changes in the rib cage and abdominal compartments, but the complexity of the muscular system makes it difficult to describe changes in the force of concentration of any particular muscle [27,28]. Therefore, OSTAP block targeting the nerves T6-L1 and causing motor block on abdominal muscles may theoretically create concern about respiratory impairment. The effects of bilateral dual TAP block on abdominal muscles was investigated by Peterson et al. [29]; they did not find any clinically significant change in spirometric respiratory function variables in healthy male volunteers.
Recently, Carrie et al. [30] reported little change in the restrictive respiratory pattern after bilateral subcostal TAP block in a patient undergoing splenectomy. Contrasting with their single case report, our study indicated that OSTAP block caused approximately 5–11% restoration of FEV1 and FVC at 2 h and 24 h after laparoscopic cholecystectomy. Although the pain scores at the postoperative 24 h between the study groups were not significantly different, patients in the OSTAP group continued to have better respiratory function and less opioid consumption. OSTAP block might increase the effectiveness of deep breathing and coughing during removal of secretions via its analgesic and opioid-sparing effects.
Limitations of the current study were that we did not make sensory block assessment to maintain the double-blind study design. However, the exact analgesic effectiveness of OSTAP block was more reliably reflected by VAS pain score and opioid consumption rather than sensory level. Secondly, the results of the current study are only valid for relatively healthy patients undergoing laparoscopic cholecystectomy. Hence, generalization of the results to ASA III and IV patients and to patients with limited respiratory function requires further studies. Finally, we did not compare the analgesic and pulmonary effects of epidural analgesia with OSTAP block. Epidural catheter placement is a relatively time-consuming procedure and may have several adverse effects, including rare severe complications. In contrast to the epidural analgesia, TAP block is a relatively safe technique with only a few case reports of significant complications [31,32]. However, further studies comparing the 2 analgesic methods would be helpful.
Conclusions
In conclusion, we observed that a single-injection OSTAP block was effective in the context of postoperative pain control, decreased opioid consumption, and improvement of respiratory functions. The observed effectiveness of OSTAP block may have favorable implications for clinically important outcomes such as pulmonary complication rates and hospitalization days. The positive effects of OSTAP block should be investigated in patients with preexisting pulmonary disease. Further trials are needed to answer these questions.
Abreviations
- TAP
transversus abdominis plane
- OSTAP
oblique subcostal transversus abdominis plane
- VAS
visual analog scale
- ECG
electrocardiography
- TOF
train of four
- ASA
American Society of Anesthesiologists
- ASA PS
American Society of Anesthesiologists Physical Status
- FVC
forced vital capacity
- FEV1
forced expiratory volume in first second
- PEFR
peak expiratory flow rate
- SO2
oxygen saturation
- PACU
postanesthesia care unit
- MAP
mean arterial blood pressure
- HR
heart rate
- PEEP
positive end-expiratory pressure
- IV
intravenous
- PO
per os
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Slinger P. From the Journal archives: Postoperative analgesia: effect on lung volumes. Can J Anaesth. 2014;61(2):200–2. doi: 10.1007/s12630-013-0085-6. [DOI] [PubMed] [Google Scholar]
- 2.Ali J, Weisel RD, Layug AB, et al. Consequences of postoperative alterations in respiratory mechanics. Am J Surg. 1974;128(3):376–82. doi: 10.1016/0002-9610(74)90176-7. [DOI] [PubMed] [Google Scholar]
- 3.Brull R, McCartney CJ, Chan VW, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104(4):965–74. doi: 10.1213/01.ane.0000258740.17193.ec. [DOI] [PubMed] [Google Scholar]
- 4.Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276–82. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 5.Staehr-Rye AK, Rasmussen LS, Rosenberg J, et al. Minimal impairment in pulmonary function following laparoscopic surgery. Acta Anaesthesiol Scand. 2014;58(2):198–205. doi: 10.1111/aas.12254. [DOI] [PubMed] [Google Scholar]
- 6.Hebbard PD, Barrington MJ, Vasey C. Ultrasound-guided continuous oblique subcostal transversus abdominis plane blockade: description of anatomy and clinical technique. Reg Anesth Pain Med. 2010;35(5):436–41. doi: 10.1097/aap.0b013e3181e66702. [DOI] [PubMed] [Google Scholar]
- 7.Maeda A, Shibata SC, Kamibayashi T, et al. Continuous subcostal oblique transversus abdominis plane block provides more effective analgesia than single-shot block after gynaecological laparotomy: A randomised controlled study. Eur J Anaesthesiol. 2014 doi: 10.1097/EJA.0000000000000167. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Shin HJ, Oh AY, Baik JS, et al. Ultrasound-guided oblique subcostal transversus abdominis plane block for analgesia after laparoscopic cholecystectomy: a randomized, controlled, observer-blinded study. Minerva Anestesiol. 2014;80(2):185–93. [PubMed] [Google Scholar]
- 9.Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 10.Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:1–100. [PubMed] [Google Scholar]
- 11.Petersen PL, Stjernholm P, Kristiansen VB, et al. The beneficial effect of transversus abdominis plane block after laparoscopic cholecystectomy in day-case surgery: a randomized clinical trial. Anesth Analg. 2012;115(3):527–33. doi: 10.1213/ANE.0b013e318261f16e. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Liu F, Tang H, et al. The analgesic efficacy of subcostal transversus abdominis plane block compared with thoracic epidural analgesia and intravenous opioid analgesia after radical gastrectomy. Anesth Analg. 2013;117(2):507–13. doi: 10.1213/ANE.0b013e318297fcee. [DOI] [PubMed] [Google Scholar]
- 13.Chen CK, Tan PC, Phui VE, et al. A comparison of analgesic efficacy between oblique subcostal transversus abdominis plane block and intravenous morphine for laparascopic cholecystectomy. A prospective randomized controlled trial. Korean J Anesthesiol. 2013;64(6):511–16. doi: 10.4097/kjae.2013.64.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joris J, Kaba A, Lamy M. Postoperative spirometry after laparoscopy for lower abdominal or upper abdominal surgical procedures. Br J Anaesth. 1997;79(4):422–26. doi: 10.1093/bja/79.4.422. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Vivien A, Sartene R, et al. Diaphragm dysfunction induced by upper abdominal surgery. Role of postoperative pain. Am Rev Respir Dis. 1983;128(5):899–903. doi: 10.1164/arrd.1983.128.5.899. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86(3):598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Egan TM, Herman SJ, Doucette EJ, et al. A randomized, controlled trial to determine the effectiveness of fascial infiltration of bupivacaine in preventing respiratory complications after elective abdominal surgery. Surgery. 1988;104(4):734–40. [PubMed] [Google Scholar]
- 18.Hendolin H, Lahtinen J, Lansimies E, et al. The effect of thoracic epidural analgesia on respiratory function after cholecystectomy. Acta Anaesthesiol Scand. 1987;31(7):645–51. doi: 10.1111/j.1399-6576.1987.tb02637.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller L, Gertel M, Fox GS, et al. Comparison of effect of narcotic and epidural analgesia on postoperative respiratory function. Am J Surg. 1976;131(3):291–94. doi: 10.1016/0002-9610(76)90119-7. [DOI] [PubMed] [Google Scholar]
- 20.Rademaker BM, Ringers J, Odoom JA, et al. Pulmonary function and stress response after laparoscopic cholecystectomy: comparison with subcostal incision and influence of thoracic epidural analgesia. Anesth Analg. 1992;75(3):381–85. doi: 10.1213/00000539-199209000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Keus F, Ahmed Ali U, Noordergraaf GJ, et al. Laparoscopic vs. small incision cholecystectomy: Implications for pulmonary function and pain. A randomized clinical trial. Acta Anaesthesiol Scand. 2008;52(3):363–73. doi: 10.1111/j.1399-6576.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 22.Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102(2):179–90. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 23.Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–1999. Anesthesiology. 2004;101(4):950–59. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Horner RL, Innes JA, Guz A. Reflex pharyngeal dilator muscle activation by stimuli of negative airway pressure in awake man. Sleep. 1993;16(8 Suppl):S85–86. doi: 10.1093/sleep/16.suppl_8.s85. [DOI] [PubMed] [Google Scholar]
- 25.Remmers JE. Wagging the tongue and guarding the airway. Reflex control of the genioglossus. Am J Respir Crit Care Med. 2001;164(11):2013–14. doi: 10.1164/ajrccm.164.11.2110043a. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, Kusuhara N, Yoshimura N, et al. Differential respiratory activity of four abdominal muscles in humans. J Appl Physiol (1985) 1996;80(4):1379–89. doi: 10.1152/jappl.1996.80.4.1379. [DOI] [PubMed] [Google Scholar]
- 27.Gauthier AP, Verbanck S, Estenne M, et al. Three-dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J Appl Physiol (1985) 1994;76(2):495–506. doi: 10.1152/jappl.1994.76.2.495. [DOI] [PubMed] [Google Scholar]
- 28.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15(4):641–59. [PubMed] [Google Scholar]
- 29.Petersen M, Elers J, Borglum J, et al. Is pulmonary function affected by bilateral dual transversus abdominis plane block? A randomized, placebo-controlled, double-blind, crossover pilot study in healthy male volunteers. Reg Anesth Pain Med. 2011;36(6):568–71. doi: 10.1097/AAP.0b013e3182330b95. [DOI] [PubMed] [Google Scholar]
- 30.Carrie C, Biais M. Subcostal TAP block and postoperative respiratory function after abdominal surgery. Anaesthesia. 2014;69(9):1056–57. doi: 10.1111/anae.12816. [DOI] [PubMed] [Google Scholar]
- 31.Singh SK, Corbett JF. Transversus abdominis plane blocks and liver injury. Br J Anaesth. 2010;104(6):782. doi: 10.1093/bja/aeq103. author reply 783. [DOI] [PubMed] [Google Scholar]
- 32.Weiss E, Jolly C, Dumoulin JL, et al. Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg Anesth Pain Med. 2014;39(3):248–51. doi: 10.1097/AAP.0000000000000088. [DOI] [PubMed] [Google Scholar]