Abstract
Background
The Eph receptors are the largest receptor tyrosine kinase family. Several family members are expressed in hematopoietic cells. Previously, the expression of a member of this family, EphA2, was identified on dendritic like cells in tonsils. We therefore specifically examined the expression of EphA2 on in vitro generated dendritic cells.
Results
In this study, expression of the EphA2 receptor was identified on in vitro generated Langerhans like dendritic cells compared to in vitro generated dendritic cells. We show that ligand induced engagement of the EphA2 receptor leads to receptor autophosphorylation indicating a functional receptor signaling pathway in these cells. We also observe phosphorylation and dephosphorylation of distinct proteins following ligand activation of EphA receptors. In co-stimulation assays, receptor-ligand interaction reduces the capacity of the Langerhans like dendritic cells to stimulate resting CD4+ T cells.
Conclusion
Engagement of EphA receptor tyrosine kinases on Langerhans like dendritic cells induces signaling as shown by tyrosine phosphorylation and dephosphorylation of distinct proteins. Furthermore this engagement renders the cells less capable of stimulating CD4+ T cells.
Background
Immature dendritic cells are localized in tissues where they monitor the microenvironment and are characterized by their capacity to take up antigens. Dendritic cells must be activated by "danger signals" to become efficient antigen presenting cells [1-3]. This maturation process includes an efficient presentation of processed antigens by inducing cell surface expression of peptide loaded MHC molecules and an increased production of cytokines. Up-regulation of specific co-stimulatory and co-adhesive molecules, like CD80 and CD86, are also necessary to fully activate T cells. Finally, a potential to migrate to the lymph nodes is developed. The mature dendritic cell is thus equipped with a package of information that orchestrates the T cell response [4]. Dendritic cells can be divided into several groups, with different cellular origins, localization and capacity to stimulate a primary T cell response [1]. One group is the Langerhans cells, which are immature dendritic cells of myeloid origin resident in squamous epithelia, including skin and mucosa. These cells are characterized by high cell surface expression of CD1a and E-cadherin, in addition to the presence of Birbeck granules with langerin [5]. Recently, it has been shown that Langerhans like cells can be generated in vitro from both adherent monocytes and CD34+ bone marrow cells in the presence of transforming growth factor β (TGF-β) [6,7].
Previously, we have investigated the expression of Eph receptor tyrosine kinases and their ligands in lymphoid tissues [8,9]. The Eph kinases are the largest known subfamily of receptor tyrosine kinases with 15 distinct members highly conserved from insects to man [10]. The Eph receptor tyrosine kinases bind a family of ligands called ephrins, consisting of two subclasses, ephrin-A and ephrin-B [10]. The six ephrin-A ligands are anchored to the membrane by a glycosylphosphatidylinositol (GPI)-tail, while the three ephrin-B ligands are transmembrane molecules. Members of both the Eph tyrosine kinases and the ephrin ligands mediate signaling after receptor-ligand interaction [11-17]. This bi-directional signaling are known to affect processes involving cellular interaction, like cell adhesion, cell migration and tissue border formation [16-18]. In particular, signaling through both the Eph kinases and the ephrin ligands have been shown to affect cellular adhesion through integrins [14,19-22]. One receptor of the Eph family, EphA2, is expressed in rat intestine and skin [23], and in fetal mouse skin and the epithelial lining of the esophagus [24]. Previously, we have shown the presence of EphA2 mRNA in several human hematopoietic tissues, and also identified protein expression in an adherent tonsil cell population with a dendritic appearance [8]. The aim of this study was therefore to identify a dendritic cell population expressing EphA2 and further investigate its functional role.
Here, we present the selective expression of EphA2 on in vitro generated Langerhans like dendritic cells. Functional signaling through EphA receptors is revealed by induced tyrosine phosphorylation and dephosphorylation of distinct proteins. Ligation of EphA receptors with a ligand reduces the capacity of Langerhans like dendritic cells to stimulate resting CD4+ T cells.
Results and Discussion
In vitro generated Langerhans like dendritic cells (LLDC) express the EphA2 receptor tyrosine kinase
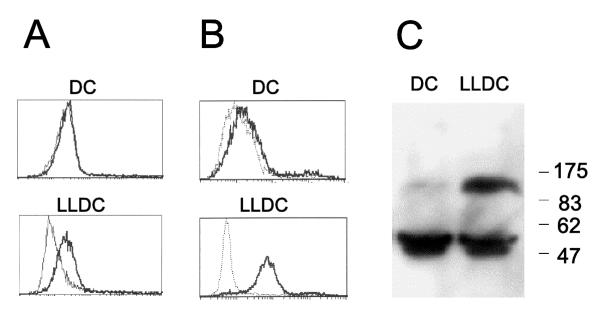
Previously, we reported the expression of the EphA2 receptor tyrosine kinase on dendritic like cells in tonsil [8], and we therefore set out to investigate the potential role for this receptor in antigen presenting cells. E-cadherin, an adhesion molecule expressed by Langerhans cells, is known to regulate both the expression and the function of the EphA2 receptor tyrosine kinase [6,25,26]. It has been shown that E-cadherin expression is required for EphA2 receptor localization at cell-cell contacts in epithelial cells. In the absence of functional E-cadherin, EphA2 does not reach the cell surface but instead localizes to the perinuclear region [25]. Therefore in vitro generated LLDC was tested for the expression of E-cadherin and the EphA2 receptor. Adherent mononuclear cells, isolated from blood, were grown for one week in the presence of GM-CSF, IL-4 and with or without TGF-β to generate LLDC and dendritic cells, respectively [6]. In line with previously published data, the LLDC expressed cell surface E-cadherin while the dendritic cells did not (data not shown). Flow cytometry analysis of cells stained with a monoclonal anti-EphA2 antibody (figure 1A) or an soluble EphA2-ligand, ephrin-A4-Fc [8] respectively, revealed significant cell surface expression of the EphA2 receptor on LLDC, and much lower expression on dendritic cells generated without TGF-β (figure 1B). In addition, total EphA2 protein expression level was assessed in the two cell populations by Western blot analysis. Comparable to the cell surface expression of EphA2, a much higher level of total EphA2 protein was observed in the LLDC compared to the dendritic cells (figure 1C).
Figure 1.
The EphA2 receptor tyrosine kinase is expressed by in vitro generated LLDC Adherent mononuclear cells were isolated and cultured one week in the presence of GM-CSF and IL-4 (for monocyte derived dendritic cells, DC) and TGF-β (for monocyte derived LLDC). (A) Cells were incubated with a monoclonal EphA2 antibody (bold line) or an irrelevant antibody (dotted line) followed by staining with PE-labeled α-mouse antibody. (B) Cells were incubated with a soluble EphA2 ligand fusion protein (ephrin-A4-Fc, bold line) and a negative control fusion protein (CD19-Fc, dotted line). (C) A Western blot with cell lysates from monocyte derived dendritic cells (DC) or LLDC (LLDC) were immunoblotted using a polyclonal anti-EphA2 antiserum.
By PCR, the expression of other EphA members in LLDC was also tested (EphA1, A2, A3, A4, A5, A7, A8). In addition to EphA2, expression of EphA1 could also be detected (data not shown). In this study, we have not investigated the expression of EphA1 at the protein level. Thus, we cannot exclude that EphA1 is involved in the functional effects observed after ligand binding presented below.
During the preparation of this manuscript, the expression of EphA2 was reported on epidermis residing Langerhans cells [27].
Ephrin-A induces signaling through EphA receptor kinases in LLDC
Several different ephrin ligands of the ephrin-A family can bind and activate the EphA2 receptor tyrosine kinase [28]. We have previously shown that both the genes encoding EphA2 and an EphA2 ligand, ephrin-A4, are expressed in several hematopoietic tissues like lymph node, spleen and fetal liver [8]. In addition, we have observed ephrin-A1 mRNA expression in tonsils (data not shown). Thus, both ephrin-A1 and ephrin-A4 might be in vivo candidate ligands for EphA receptors expressed on Langerhans cells. We have previously shown that T cells express ephrin-A4 mRNA after anti-CD3 stimulation [8]. Further, we also show here that expression of ephrin-A4 protein is induced on CD4+ T cells stimulated with anti-CD3/CD28 Dynabeads, and reaches maximal expression after three days of stimulation (figure 2). Thus, ephrin-A4 expressed on activated T cells might be a physiological ligand for EphA receptors expressed on LLDC.
Figure 2.
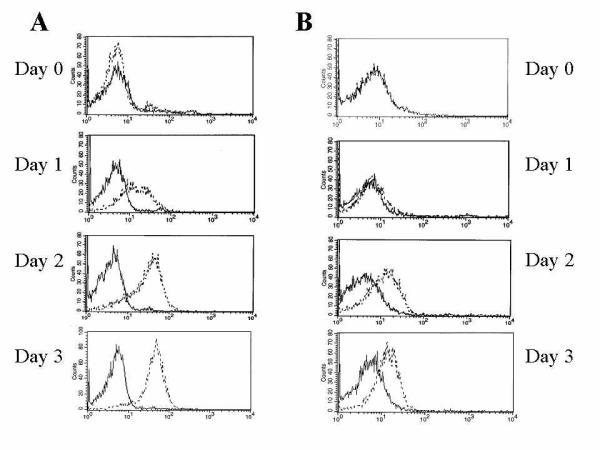
Expression of ephrin-A4 on CD4+ T cells Isolated CD4+ T cells were stimulated with anti-CD3/CD28 Dynabeads for the indicated times before staining with an anti-ephrin-A4 antibody (panel A) or EphA2-Fc (panel B). 10000 cells were analyzed. Highest expression of cell surface bound ephrin-A4 was observed after three days of stimulation.
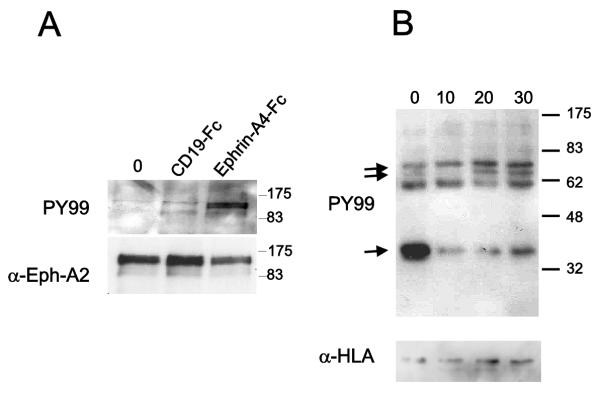
The first event that takes place after interaction between an Eph receptor and its ligand is autophosphorylation of the intracellular part of the Eph receptor. To validate the functionality of the receptor-ligand interaction, the phosphorylation level of EphA2 after ephrin-A4 cross-linking was investigated. LLDC were incubated with immobilized ligand (ephrin-A1-Fc) or a control fusion protein (CD19-Fc) for ten minutes at 37°C. The cells were then lysed and the EphA2 receptor was immunoprecipitated with a polyclonal anti-EphA2 antiserum. The phosphorylation status of the receptor was analyzed using a anti-phosphotyrosine specific antibody. In agreement with previous studies [28], tyrosine phosphorylation of EphA2 was observed after cross-linking with ephrin-A1 (figure 3A). The filter was stripped and re-probed with anti-EphA2 antiserum to confirm equal loading of proteins (figure 3A). Phosphorylation of EphA2 was also seen after incubation with ephrin-A4-Fc (data not shown). The results show that the EphA2 receptor expressed on LLDC is functional and respond by auto-phosphorylation after ligand activation.
Figure 3.
The ephrin-A4 ligand induces signaling through EphA receptors A. Serum starved LLDC were left untreated (untreated) incubated with immobilized ephrin-A4-Fc or a control protein (CD19-Fc) for 10 minutes. The EphA2 receptor was immunoprecipitated, and the phosphorylation status of the EphA2 kinase was analyzed by Western blot analysis with an anti-phosphotyrosine antibody (upper panel). The blot was stripped and reprobed with a polyclonal anti-EphA2 antibody (lower panel). B. Serum starved LLDC were incubated with immobilized ephrin-A1-Fc for 10, 20, and 30 min, and cell lysates were fractioned in cytosol and membrane fraction. Shown in the figure are gel-separated proteins from the membrane fraction hybridized with anti-phosphotyrosine antibody (PY99; upper panel). Arrows indicate phosphorylation or dephosphorylation of distinct proteins during the time course. The filter was stripped and hybridized with an anti-HLA antibody to verify protein loading (lower panel).
To further investigate the signaling capacity of EphA receptors, cells were incubated with bead coupled ephrin-A1-Fc for 10, 20 and 30 min, and then fractionated into cytosol and membrane fractions followed by SDS-PAGE separation, blotting and hybridization with an anti-phosphotyrosine antibody. Distinct differences in the protein tyrosine phosphorylation pattern were observed after ephrin-A1-Fc receptor ligation in the membrane fraction (figure 3B). In particular, a protein with approximately molecular mass of 70 kDa was phosphorylated upon 20 and 30 min incubation with ephrin-A1, while dephosphorylation of a protein with approximately molecular mass of 35 kDa was observed already after 10 min incubation (figure 3B). Several proteins have been reported to be phosphorylated after ephrin ligation of EphA receptors like Fak, paxillin and p130Cas [29], or dephosphorylated like Fak and paxillin [30,31]., depending on the cell system applied. None of these proteins correlate with the estimated molecular mass of the phosphorylated and dephosphorylated proteins identified in LLDC (figure 3B). The transient phosphorylation and dephosphorylation patterns observed after stimulation of Eph receptors involve distinct phosphatases. Shp-2 can associate with EphA2 and might be involved in dephosphorylation of FAK and paxillin leading to dissociation of the EphA2-FAK complex [30]. Low-molecular-weight phosphotyrosine phosphatase (LMW-PTP) can be recruited to EphB2 receptor complexes after ephrin ligation [31]. Whether these phosphatases are involved in EphA signaling in LLDC awaits further studies.
Engagement of Eph receptors does not influence the cell surface expression of accessory- and adhesion molecules of LLDC
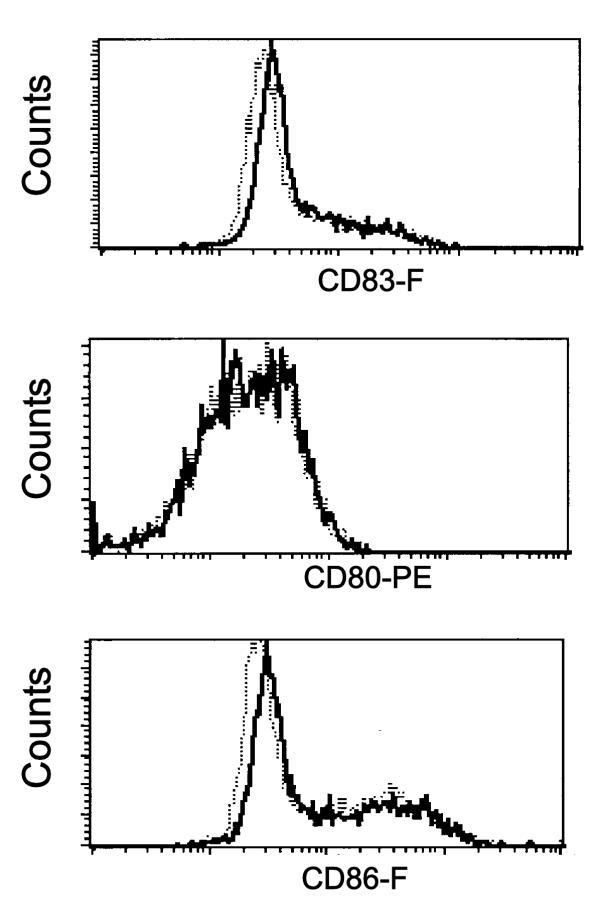
Interaction between an antigen presenting cell (APC) and a responding T cell induce signaling inside the APC, in addition to activation of the T cells. Although there is detailed information about the functional signaling response in the T cell after contact with an APC presenting specific antigen, less is known about the consequences for the APC. TRANCE and CD40L present on the CD4+ T cell are known to induce a survival signal for the APC and license the APC to activate CD8+ T cells, respectively [33-36]. The interaction of antigen specific CD8+ T cells may also induce a response in the dendritic cell, as measured by IL-12 production by the dendritic cells [37]. One may therefore speculate whether signaling through EphA kinases may interfere with the T cell induced maturation of the dendritic cell. To investigate whether EphA receptor signaling affects the maturation status of the LLDC, the cells were cultured for 18 hours in the presence of immobilized ephrin-A4-Fc or negative control fusion protein (CD19-Fc). Cell surface expression of accessory and differentiation/activation molecules (CD18, CD11a, CD11c, CD49d, CD80, CD86, CD83, HLA-DR, CD40, CD58 and E-Cadherin) was analyzed by flow cytometry. None of these molecules showed differences in the expression pattern between cells grown on immobilized ephrin-A4-Fc or CD19-Fc (figure 4 and data not shown).
Figure 4.
Cell surface expression of activation markers on monocyte derived LLDC after EphA receptor engagement LLDC were cultured 18 hours in the presence of immobilized ephrin-A4-Fc (bold lines) or the negative control protein CD19-Fc (dotted lines). Cell surface expressions of the indicated molecules were detected by flow cytometry analysis. 10000 cells were analyzed.
Effect of engagement of EphA receptors on LLDC on the proliferative response of T cells in co-culture assays
Based on the observation that EphA activation led to changes in the tyrosine phosphorylation pattern; we investigated if EphA receptor ligation had any consequence for the capacity of the LLDC to activate T cells. An allogeneic assay was performed with increasing numbers of LLDC or dendritic cells in co-culture with 50 000 CD4+ T cells in the presence of either immobilized ephrin-A4-Fc or CD19-Fc (control protein). T cell proliferation was measured as thymidine uptake on day 6–7. For this experiment ephrin-A4 was chosen since we observed binding of ephrin-A1-Fc to T cells, as shown by others, but only weak or no binding of ephrin-A4-Fc (data not shown).
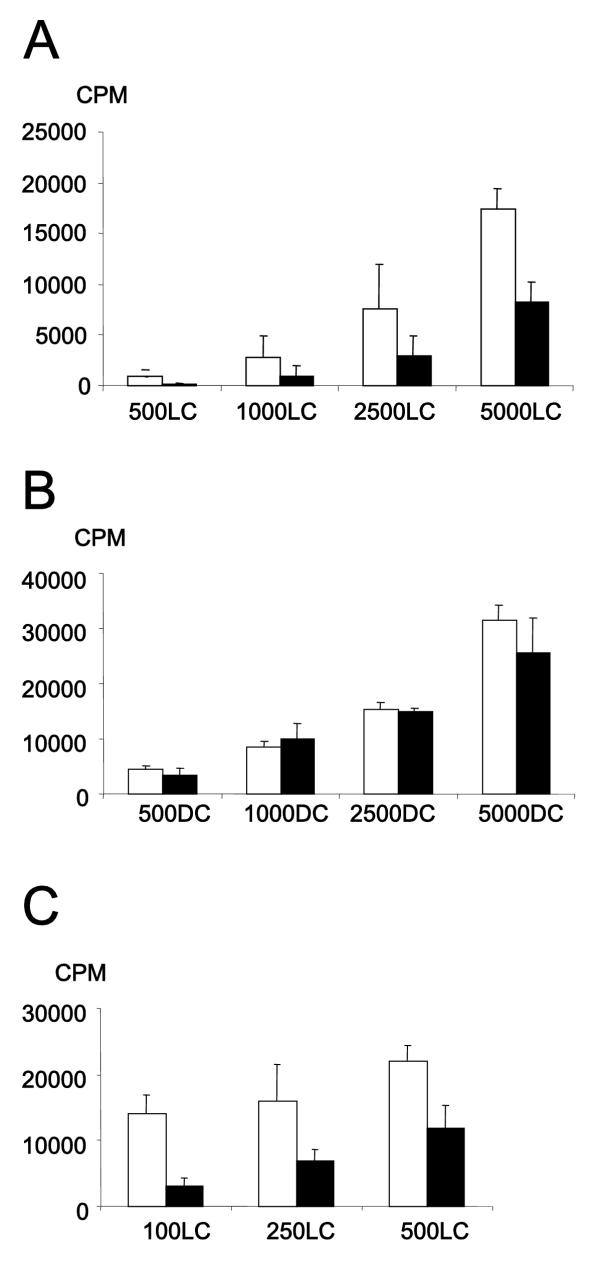
In a co-culture with LLDC and CD4+ T cells, the presence of immobilized ephrin-A4 resulted in reduced T cell proliferation (figure 5A). Soluble ephrin-A4-Fc had no effect on T cell proliferation (data not shown). The effect was most pronounced at a high T cell: LLDC ratio. Important, for dendritic cells (generated without TGF-β), grown with or without immobilized ephrin-A4, no differences in CD4+ T cell proliferation were observed (figure 5B) excluding a direct effect through T cells.
Figure 5.
Signaling through EphA receptors reduces the stimulatory capacity of LLDC to activate CD4+T cells The indicated numbers of antigen presenting cells were used to stimulate 50000 CD4+ cells in the presence of immobilized control fusion protein (CD19-FC, open columns) or ephrin-A4-Fc (filled column). T cell proliferation was measured by 3H-thymidine incorporation on day 6–7. One of at least three representative experiments is shown here, the values are the mean of triplicates with the SD indicated. (A) An allogeneic presenting assay with LLDC (LLDC). (B) An allogeneic presenting assay with monocyte derived dendritic cells (DC). (C) An autologous antigen-presenting assay with LLDC (LLDC) pulsed with tuberculosis PPD antigen.
Also a syngeneic antigen presentation assay, using the recall antigen PPD from mycobacterium, was performed. Increased numbers of LLDC, on either immobilized ephrin-A4-Fc or CD19-Fc, were co-cultured with 50000 CD4+ T cells in the presence of PPD. Also here, ligation of EphA receptors with immobilized ephrin-A4 resulted in reduced T cell proliferation indicating that the reduction in the stimulatory capacity of the LLDC is independent of the presented antigen. As shown for the allogeneic assay (figure 5A), the effect was most pronounced at a high T cell: LLDC ratio (figure 5C). Thus, the reduction in T cell proliferation is most pronounced when sub-optimally stimulating the T cell.
Dendritic cells strictly regulate the quantity of a T cell response. Adhesion molecules are involved in the synapse formation and adhesion between antigen presenting cells and T cells influence the activation. In addition, rearrangements of the actin cytoskeletal in dendritic cells are necessary for optimal stimulation of resting T cells [38]. Bi-directional signaling through the Eph receptors and ephrin ligands influence both the shape and the adhesive properties of cells, by affecting integrin affinity and the cytoskeleton [16,18]. In addition, MAPK activation has been shown to result in inhibition of adhesion to extracellular matrix [32]. MAPK activation has also been shown to influence activation and maturation of dendritic cells [39,40]. Thus, one may speculate that a reduced contact between the LLDC and the T cell, possibly through integrin deactivation, inhibits stimulation of T cells, although we did not observe any difference in expression of β2 integrins (CD18, CD11a and CD11c). In contrary, a recent report presents results that indicates increased adhesion of EphA2 expressing dendritic cells derived from CD34+ progenitors to fibronectin coated surfaces in the presence of ephrin-A3 through β1 integrin activation [27].
Alternatively, the activation of Eph kinases induces translocation to caveolae-like domains, thus; engagement of EphA receptors on LLDC with immobilized ligand may induce ectopic raft aggregates, and thereby destabilize the immunological synapse [41,42]. We have not tested if secretions of soluble factors important for T cell activation are affected after EphA receptor activation on LLDC. Thus we cannot exclude that altered secretion might lead to the observed reduced T cell proliferation.
Cross-linking of EphA receptors on LLDC does not change the cytokine profile indicative of a Th1 or Th2 response
On the background of the results presented in figure 5, we found it interesting to examine the functional quality of the T cells resulting from co-culture. A reduced number of the re-stimulated T cells expressed the activation marker CD25 when the T cells where co-cultivated in the presence of immobilized ephrin-A4-Fc compared to the CD19-Fc (table 1). The effect is most pronounced when suboptimal stimulating the T cells with a high T cell: LLDC ratio (table 1). This is in accordance with the inhibition of proliferation measured by thymidine incorporation (figure 5A,5C).
Table 1.
Expression of differentiation and activation markers on re-stimulated CD4+ cells
| IFN-g, % pos | IL4, % pos | CD25, % pos | |
| CD19-Fc, 1:100 | 3.0 | 2.3 | 10.3 |
| ephrin-A4-Fc, 1:100 | 1.9 | 1.4 | 6.1 |
| CD19-Fc, 1:10 | 10.2 | 2.6 | 19.3 |
| ephrin-A4-Fc, 1:10 | 7.4 | 2.1 | 17.3 |
The reduction in T cell proliferation is not a result of a change in the Th1/Th2 ratio, but rather a general reduction in the activation status of the T cells. Resting CD4+ cells were stimulated one week with LLDC, in the presence of immobilized control protein or ligand, and re-stimulated 5 hours with TPA and ionomycin. One of three representative experiments is shown, and data represent percent of living cells staining the indicated antibody.
Functional activation of CD4+ T cells induces the expression of cytokines indicative for Th1 or Th2 responses. Different factors such as the number and type of antigen presenting cells, co-stimulatory molecules and the duration of T cell receptor stimulation influence the polarization of the T cell response, although cytokines are the dominant regulators [43,44]. To investigate if EphA ligation had a selective effect on polarization of effector cell, autologous CD4+ T cells were cultured one week with LLDC in the presence of either immobilized ephrin-A4-Fc or CD19-Fc, before re-stimulation with TPA and ionomycin. The T cells were then analyzed for the expression of cytokines indicative of either a Th1 (IFN-γ) or a Th2 response (IL-4) by flow cytometry. Although EphA receptor ligation reduced the number of T cells expressing the indicated cytokines on a general basis compared to CD19-Fc, no selective reduction in either of the Th1 or Th2 populations were seen (table 1). This demonstrates that EphA receptor signaling in LLDC does not influence these functional properties of CD4+ T cells.
Since the reduction in proliferation of CD4+ T cells is more pronounced at a high CD4+: LLDC ratio, the differentiation status of these cells in a mixed lymphocyte reaction was also studied with higher numbers of LLDC. In accordance with what is reported in the literature, we observed in our system an increased number of T cells producing cytokines, especially the Th1 cytokine IFN-γ, when decreasing the CD4+: LLDC ratio (table 1). In conclusion, engagement of EphA receptors on LLDC does not seem to affect the polarization of CD4+ T cells, which may indicate that the effect is through inhibition of adhesion.
Conclusions
The presence of several Eph receptor tyrosine kinases and ephrin ligands in cells involved in immune responses suggest a functional role for these genes in immunity [8,41,45,46]. However, the molecular mechanism still remains elusive. Here we specifically present expression of the EphA2 receptor tyrosine kinase on monocyte derived LLDC. The activation of EphA receptor tyrosine kinases on these cells reduces the potential of monocyte derived LLDC to activate CD4+ T cells. The outcome of signaling through Eph receptors has been demonstrated to be either increased adhesion or decreased adhesion dependent on the cell system studied. Although the mechanism for the reduced T cell proliferation remains unknown, one might speculate that it is due to a reduced interaction between the LLDC and T cells, or that EphA ligation inhibits activation and maturation of the LLDC. CD4+ T cells acquire an Eph ligand, ephrin-A4, after stimulation with CD3/CD28, and thus these cells might interact with Langerhans cells in vivo e.g. in lymph nodes through this ligand. Interestingly, several Eph receptors and ephrin ligands are ectopically expressed in cancers, e.g. both EphA2 and ephrin-A1 are over-expressed in aggressive melanomas [47,48]. It is tempting to speculate that the influence of Eph and ephrin interactions in immunity play a role in the etiology of cancer, in addition to the increased angiogeneic potential and the effect on migration of metastatic cells [49].
Methods
Antibodies, cytokines and fusion proteins
Monoclonal anti-EphA2 was a kind gift from Dr. R.A Lindberg [23] and polyclonal α-EphA2 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphotyrosine antibodies: PY99 (Santa Cruz) and 4G10 (Upstate Biotechnology, Lake Placid, NY). Secondary antibody for Western blot hybridization: α-mouse-HRP (DAKO, Copenhagen, Denmark). Antibodies used for flow cytometry analysis: α-CD25 (DAKO), α-CD80 (Becton Dickinson, SanJose, CA), α-CD83 (Immunotech, Marseilles, France), α-CD86 (PharMingen, San Diego, CA), α-HLA-DR (Becton Dickinson), α-E-Cadherin (Zymed Laboratories Inc, San Francisco, CA), phycoerytrin-labeled (PE) – or Fluorescein (FITC)-labeled (F) α-mouse Ig polyclonal antibody (Ig-RPE and Ig-RF, Southern Biotechnology Associates, Birmingham, AL, USA), α-IL-4-PE and α-INFγ-FITC (PharMingen). IL-4 was a kind gift from Schering-Plough Research Institute (Kenilworth, J), TGF-β1 was obtained from Habersham Pharmacia Biotech (Uppsala, Sweden) and GM-CSF was obtained from Roche (Mannheim, Germany). Fusion proteins of the extracellular part of either CD19 (negative control protein) or ephrin-A4 fused to the mouse constant and hinge region of IgG2b heavy chain is previously described in [8]. Ephrin-A1-Fc fusion protein was generated as described in [8], with gene specific primers.
Cell isolation and treatments
Mononuclear cells were obtained from Buffy coat from normal, healthy donors using the lymph prep kit (Nycomed Pharma, Oslo, Norway). Platelet numbers were reduced by 8 min. centrifugation at 180 g. 20 × 106 mononuclear cells were seeded per well in a 6-well plate (Costar Corp., Cambridge, MA), and non-adherent cells were removed by extensive washing after incubation at 37°C for two hours. Cells were grown for one week in RPMI 1640, supplemented with 10% FCS, 200 ng/ml GM-CSF and 100 ng/ml IL-4. For the generation of LLDC, 10 ng/ml rhTGF-β1 were added in addition. Fresh medium supplemented with cytokines was added at day 2–3. Any remaining B and T cells were depleted with anti-CD19 and anti-CD3 coated Dynabeads (Dynal Biotech, Oslo, Norway) before experiments with LLDC. CD4+ T cells were isolated using anti-CD4 coated Dynabeads (Dynal) and resting CD4+ T cells were obtained using negative depletion with anti-MHC class II Dynabeads (Dynal). CD3/CD28 stimulation of CD4+ T cells was performed with 2 Dynabeads (Dynal) per T cell. All cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5 % CO2.
Antigen presentation assays
Round-bottom 96-well tissue culture plates were coated with 10 ug/ml α-mouse-IgG in 50 mM Tris-HCl pH 9.5, blocked with 0.1% BSA, and finally incubated with 20 ug/ml of the indicated fusion proteins in PBS. Indicated numbers of dendritic cells were seeded along with 50000 CD4+ cells in RPMI-1640 medium supplemented with 5% FCS and antibiotics. In addition, for autologue presentation assay, 2 ng/ml PPD ("purified protein derivative") from Mycobacterium tuberculosis (Veterinary Institute, Oslo, Norway) were added. At day 6–7, the cells were pulsed with 3.7 × 104 Bq/well 3H-thymidine ON. The cells were harvested in a 96-well harvester (Packard, Meriden, CT), and incorporated thymidine was measured with a Top Count liquid scintillation counter (Packard). All experiments were performed in triplicate.
Subcellular fractionation
LLDC were stimulated with ephrin-A1-Fc for the indicated times, then washed in ice-cold phosphate-buffered saline (PBS) and resuspended in buffer A (5 mM Tris-HCl, pH 8,0.5 mM EDTA, 75 mM sucrose and proteinase inhibitors) and sonicated four times 15 s. Nuclei were pelleted by centrifugation at 400 g for 5 min 4°C in a microsentrifuge. The supernatants were centrifuged at 32 000 g for 30 min at 4°C in a Beckmann centrifuge. The supernatants was collected and used as the cytosol fraction. The membrane pellets were washed three times with PBS, solubilised in buffer A containing 1 % Triton X-100 for 15 min at 4°C and then centrifuged at 10 000 g for 10 min at 4°C in a microsentrifuge. Supernatants were used as the membrane fraction.
Phosphorylation, immunoprecipitation and Western blot
LLDC were serum starved (1% FBS) over night and washed in PBS. Prewarmed cells were then incubated with Ephrin-A1-Fc coated on anti-mouse Ig magnetic beads (five beads/cell; Dynal, Oslo, Norway) for the indicated times at 37°C before resuspension in buffer A, sonication and fractionation into cytosol and membrane fractions as described above (subcellular fractionation) or lysis in lysis buffer (PBS, 1% NP40, aprotinin (Sigma, St.Louis, MO) and 0.5% phosphatase inhibitor cocktail II (Sigma) on ice for 30 minutes. Protein concentrations were estimated by Ponceau red (Sigma) staining of dot blots on nitrocellulose membranes (Schleicher and Schuell GmbH, Dassel, Germany) by comparison with proteins of known concentration. 1 ug polyclonal antibody was used to precipitate the EphA2 receptor over night at 4°C, and the antibody-complex was captured with protein-G sepharose (Pharmacia). After three times washing with TBS + 0.1% Tween20, captured proteins were eluted by boiling in 3 × SDS sample buffer. PAGE was performed with indicated lysates or immunoprecipitates. The Western blot was immunoblotted with indicated antibodies and visualized (ECL+, Amersham). The filter was stripped and reprobed with polyclonal α-EphA2.
Cell staining and flow cytometry analysis
Cell surface staining
Cells were pre-incubated with 0.1 mg/ml human aggregated gamma globulin to block unspecific staining, then incubated with 20 ug/ml fusion protein or antibody for 30 min. at 4°C. The cells were washed twice, and stained with the indicated anti-mouse conjugated secondary antibody. 10000 cells were analyzed.
Intracellular staining
CD4+ T cells co-incubated with LLDC in the presence of immobilized fusion protein were re-stimulated with 2,5 ug/ml TPA and 250 ng/ml ionomycin for 5 hour, and 10 ug/ml brefeldinA (Sigma) was added the last 2–3 hours. Intracellular staining was performed using the Fix and Perm cell permeabilization kit (Caltag laboratories), as described by the manufacturer. 104 cells were analyzed by a FACSCalibur flow cytometer, and the analyses were performed with CELLQuest software (Becton Dickinson). 10000 cells were analyzed.
Authors' contributions
EM participated in the cell isolation and treatment; the antigen presentation assays, performed the flow cytometry analysis and drafted the manuscript. EF performed EpHA2 activation and Western blot analysis. HCA participated in the cell isolation and treatment; the antigen presentation assays and carried out the phosphorylation assay. All authors designed the study and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Schering-Plough Research for kindly providing the IL4, Dr. R.A. Lindberg for providing monoclonal EphA2-antibody, Helena Hauge for expert technical assistance and Professor Steinar Funderud for critically reading the manuscript. This work was supported by the Norwegian Research Council and the Norwegian Cancer Society.
Contributor Information
Else Munthe, Email: else.munthe@labmed.uio.no.
Eivind Farmen Finne, Email: e.f.finne@labmed.uio.no.
Hans-Christian Aasheim, Email: h.c.asheim@labmed.uio.no.
References
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- McLellan AD, Brocker EB, Kampgen E. Dendritic cell activation by danger and antigen-specific T-cell signalling. Exp Dermatol. 2000;9:313–322. doi: 10.1034/j.1600-0625.2000.009005313.x. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- Jakob T, Udey MC. Epidermal Langerhans cells: from neurons to nature's adjuvants. Adv Dermatol. 1999;14:209–258. [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J Immunol. 1999;163:4869–4877. [PubMed] [Google Scholar]
- Aasheim HC, Munthe E, Funderud S, Smeland EB, Beiske K, Logtenberg T. A splice variant of human ephrin-A4 encodes a soluble molecule that is secreted by activated human B lymphocytes. Blood. 2000;95:221–230. [PubMed] [Google Scholar]
- Munthe E, Rian E, Holien T, Rasmussen A, Levy FO, Aasheim H. Ephrin-B2 is a candidate ligand for the Eph receptor, EphB6. FEBS Lett. 2000;466:169–174. doi: 10.1016/S0014-5793(99)01793-7. [DOI] [PubMed] [Google Scholar]
- Eph Nomenclature Committee Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/S0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner K, Klein R. Signaling by Eph receptors and their ephrin ligands. Curr Opin Neurobiol. 1998;8:375–382. doi: 10.1016/S0959-4388(98)80064-0. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci STKE. 2001;2001:RE20. doi: 10.1126/stke.2001.112.re20. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Zipursky SL. Signaling downstream of Eph receptors and ephrin ligands. Cell. 2001;105:701–704. doi: 10.1016/S0092-8674(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Becker E, Huynh-Do U, Holland S, Pawson T, Daniel TO, Skolnik EY. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20:1537–1545. doi: 10.1128/MCB.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci U S A. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J, Drescher U. An ephrinA-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120 kDa protein. J Biol Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990;10:6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Pandey A, O'Shea KS, Seldin M, Dixit VM. Characterization of B61, the ligand for the Eck receptor protein-tyrosine kinase. J Biol Chem. 1995;270:5636–5641. doi: 10.1074/jbc.270.15.8837. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- Saint-Vis B, Bouchet C, Gautier G, Valladeau J, Caux C, Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood. 2004;102:4431–4440. doi: 10.1182/blood-2003-02-0500. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/S0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Iijima N, Iwabuchi K, Onoe K. Activation of extracellular signal-related kinase by TNF-alpha controls the maturation and function of murine dendritic cells. J Leukoc Biol. 2002;71:125–132. [PubMed] [Google Scholar]
- Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–3872. [PubMed] [Google Scholar]
- Luo H, Yu G, Wu Y, Wu J. EphB6 crosslinking results in costimulation of T cells. J Clin Invest. 2002;110:1141–1150. doi: 10.1172/JCI200215883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- Glimcher LH. Lineage commitment in lymphocytes: controlling the immune response. J Clin Invest. 2001;108:S25–S30. [PubMed] [Google Scholar]
- Luo H, Wan X, Wu Y, Wu J. Cross-Linking of EphB6 Resulting in Signal Transduction and Apoptosis in Jurkat Cells. J Immunol. 2001;167:1362–1370. doi: 10.4049/jimmunol.167.3.1362. [DOI] [PubMed] [Google Scholar]
- Munoz JJ, Alonso C, Sacedon R, Crompton T, Vicente A, Jimenez E, Varas A, Zapata AG. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J Immunol. 2002;169:177–184. doi: 10.4049/jimmunol.169.1.177. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Hill SP, Hsu MY, Fallowfield ME, Florenes VA, Herlyn M, Bennett DC. Up-regulation of ephrin-A1 during melanoma progression. Int J Cancer. 1999;84:494–501. doi: 10.1002/(SICI)1097-0215(19991022)84:5<494::AID-IJC8>3.3.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Guthrie BA, Maung K, Farr CJ, Lindberg RA, Toso RJ, Herlyn M, Bennett DC. Protein B61 as a new growth factor: expression of B61 and up-regulation of its receptor epithelial cell kinase during melanoma progression. Cancer Res. 1995;55:2528–2532. [PubMed] [Google Scholar]
- Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]