Abstract
A patient with end-stage renal disease (ESRD) on hemodialysis presented with fever, anorexia, and nausea shortly after starting oral lanthanum carbonate for phosphate control. Gastric and duodenal biopsies demonstrated diffuse histiocytosis with intracellular aggregates of basophilic foreign material. Transmission electron microscopy, an underutilized diagnostic test, revealed the nature of the aggregates as heavy metal particles, consistent with lanthanum. Symptoms and histiocytosis improved after discontinuation of lanthanum. Lanthanum may be an underdiagnosed cause of gastrointestinal histiocytosis.
Introduction
Patients with end-stage renal disease (ESRD) are unable to adequately excrete phosphate, putting them at increased risk for cardiovascular mortality and metabolic bone disease.1 Optimal phosphate levels in dialysis patients cannot be achieved with dietary restriction, so oral phosphate binders are used.1,2 Lanthanum carbonate is a potent, non-calcium, non-aluminum phosphate binder.3 Because of its reported safety profile and tolerability, lanthanum use is increasing.2-4
Case Report
A 64-year-old white man with ESRD on hemodialysis presented with several months of daily low-grade fever, nausea, and anorexia. He also had 4-5 daily episodes of non-bloody diarrhea (controlled with loperamide), abdominal cramps, and mild weight loss. He denied early satiety, dysphagia, odynophagia, food intolerance, or recent dietary changes. He had no sick contacts or recent travel. His past medical history included ESRD secondary to hypertension and IgA nephropathy, peripheral neuropathy, recurrent deep vein thrombosis, and a remote (>30 years ago) ileal resection for presumed ileitis with no subsequent history to suggest inflammatory bowel disease. Two years prior to presentation, the patient underwent distal pancreatectomy and splenectomy for a malignant low-grade pancreatic neuroendocrine tumor with clear surgical margins and no evidence of recurrence on serial follow-up imaging studies. His medications included aspirin, warfarin, lisinopril, gabapentin, vitamin B12, and folate. For phosphate control, he took calcium acetate and lanthanum carbonate.
The patient denied alcohol or drug abuse. The patient was febrile (temperature 38.3°C), appeared well, had a healed abdominal scar, but an otherwise unremarkable physical exam. White blood count was 6800/µL with 21% monocytes, hematocrit 39%, mean corpuscular volume 103.7 fL, and platelet count 181,000/µL. Serum electrolytes and liver function tests were normal. The erythrocyte sedimentation rate was 32 mm/hr and C-reactive protein level was undetectable.
Laboratory tests for infections included negative serum polymerase chain reaction (PCR) for Cytomegalovirus, Epstein-Barr virus, and human herpes virus 6. HIV antibody, antinuclear antibody titer, and multiple blood cultures were negative. Transthoracic echocardiogram and PET/CT scan were unremarkable. Octreotide scan was negative. Chromogranin A was increased at 1610, but had been stable and was attributed to renal impairment.5
Esophagogastroduodenoscopy (EGD) showed diffuse patchy inflammation with associated scattered gastric ulcerations. Colonoscopy showed an intact ileocolonic anastomosis, normal ileum, normal colonic mucosa, and grade 2 internal hemorrhoids. Biopsies showed histiocyte aggregates and infiltrates in the small bowel, stomach, and distal esophagus. Biopsies stained positive for CD68 (a macrophage marker) but negative for S100, CAM5.2/AE1, synaptophysin, chromogranin, and periodic acid-Schiff staining. Additional testing for Tropheryma whipplei DNA by PCR was negative. Acid-fast bacilli staining was negative for mycobacteria. Giemsa stains were negative for Helicobacter organisms. Staining for fungal organisms was negative. Focal villous blunting in the small bowel was noted; anti-tissue transglutaminase IgA was negative with normal IgA levels. Immunohistochemical kappa and lambda protein stains performed showed polytypic plasma cells without immunoglobulin crystal deposition.
Repeat EGD with gastric and duodenal biopsies again revealed scattered ulcerations (Figure 1) and a pronounced histiocytic infiltrate containing variably dense and granular deposits (Figure 2). Immunohistochemical stains for CD1a and S100 were negative, arguing against Langerhans histiocytosis. Ultrastructural studies to further characterize the histiocytic deposits revealed numerous electron-dense precipitates, which appeared predominantly lysosome-based, granular in structure, and characteristic of heavy metal deposition. The appearance of these structures was consistent with published descriptions of lanthanum in phagocytic cells grown in vitro.
Figure 1.
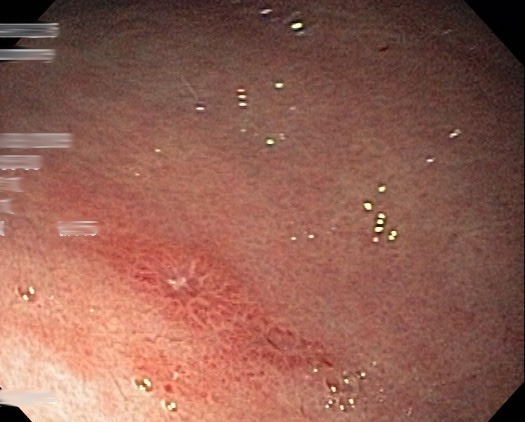
Endoscopy showing a small gastric antral ulcer due to histiocytosis.
Figure 2.
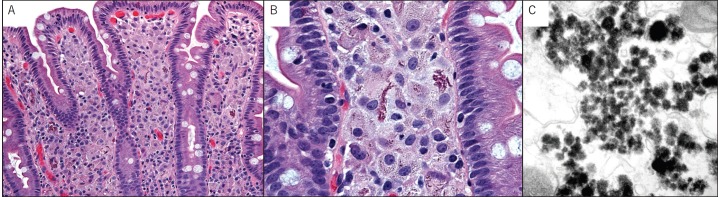
(A) Villous blunting secondary to duodenal histiocytosis. (B) Aggregates of foreign amphophilic material fill most of the histiocytes. (C) Ultrastructal demonstration of lyzosomal aggregates with structural characteristics of heavy metal.
The patient was advised to discontinue lanthanum, and his fevers and gastrointestinal symptoms resolved within 1 month. Repeat EGD 3 months after discontinuation of lanthanum revealed a normal esophagus, mild gastric erythema, and normal duodenum. Histologic sections showed resolution of the histiocytosis in the stomach and significant decrease in the duodenum (Figure 3).
Figure 3.
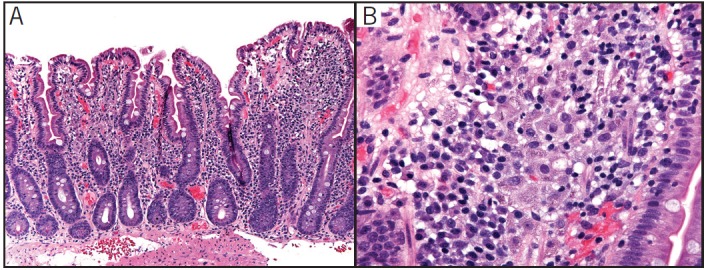
Small intestine following discontinuation of lanthanum showing (A) fewer histiocytes in the lamina propria and (B) markedly decreased intracellular aggregates of foreign material.
Discussion
This is the first case describing lanthanum-induced gastrointestinal histiocytosis, an adverse response that may arise with chronic lanthanum. This was reversible, as discontinuation of lanthanum led to symptomatic and histologic improvement. Given the prevalence of ESRD and the increasing use of lanthanum, our diagnosis is important. Only a fraction of ESRD patients undergo extensive work-up of symptoms such as nausea or anorexia, which are frequently attributed to uremia. Furthermore, the diagnosis would have been missed without electron microscopy; thus, this test should be considered when gastrointestinal histiocytosis is seen.
Initial and subsequent endoscopic biopsies revealed that lanthanum-filled histiocytes were found in the proximal GI tract. This can be explained by increased luminal lanthanum concentrations in the upper GI tract, resulting in increased absorption.3 Studies have shown that patients with ESRD on hemodialysis are at increased risk of intestinal tight junction disruption secondary to increased levels of urea.6 This could potentiate absorption of lanthanum into the submucosa. In animal studies, lanthanum has been shown to permeate between intestinal epithelial cells where it could gain access to and accumulate in subepithelial phagocytic cells. Oral ingestion of lanthanum leads to infiltration of macrophages containing the element in the small bowel of rats.7 Lanthanum's ability to diffuse from the extracellular space and enter cells makes it useful in electron microscopy studies for histiocytes.8,9
The effects of lanthanum on the reticuloendothelial system and macrophages are complex and have not been well studied. There may be similarities between the effects of lanthanum and gadolinium, another member of the lanthanide family with similar physicochemical reactivity. Gadolinium has been implicated in the pathogenesis of nephrogenic systemic fibrosis, a condition characterized by macrophage activation. Like lanthanum, gadolinium precipitates free calcium and phosphate, leading in turn to phagocytosis by macrophages.10-12 Since our patient's fever resolved after stopping lanthanum, the fever may have been due to cytokines released by the macrophages in response to the lanthanum. Further research on this topic is needed to define the prevalence and possible long-term effects.
Disclosures
Author contributions: ME Rothenberg, H. Araya, and PJ Pasricha wrote the manuscript. TA Longacre took the micrographs. ME Rothenberg and PJ Pasricha are the article guarantors.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1. Persy VP, Behets GJ, De Broe ME, D'Haese PC.. Management of hyperphosphatemia in patients with end-stage renal disease: Focus on lanthanum carbonate. Int J Nephrol Renovasc Dis. 2009;2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Zhang YX, Yu XQ, et al. . Lanthanum carbonate for the treatment of hyperphosphatemia in CKD 5D: Multicenter, double blind, randomized, controlled trial in mainland China. BMC Nephrol. 2013;14(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damment SJ, Pennick M.. Clinical pharmacokinetics of the phosphate binder lanthanum carbonate. Clin Pharmacokinet. 2008;47(9):553–63. [DOI] [PubMed] [Google Scholar]
- 4. Joy MS, Finn WF.. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42(1):96–107. [DOI] [PubMed] [Google Scholar]
- 5. Bech PR, Ramachandran R, Dhillo WS, et al. . Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine- and amphetamine-regulated transcript. Clin Chem. 2012;58(5):941–3. [DOI] [PubMed] [Google Scholar]
- 6. Vaziri ND, Yuan J, Norris K.. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyagawa M, Daimon T.. A histochemical study of lanthanum in the intestine of the rats after oral administration of lanthanum chloride in drinking water. Biomed Res Trace Elements. 2008;19(4):330–335. [Google Scholar]
- 8. Leeson TS, Higgs GW.. Lanthanum as an intracellular stain for electron microscopy. Histochem J. 1982;14(4):553–60. [DOI] [PubMed] [Google Scholar]
- 9. Cancilla PA. Demonstration of the Langerhans granule by lanthanum. J Cell Biol. 1968;38(1):248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perazella MA. Tissue deposition of gadolinium and development of NSF: A convergence of factors. Semin Dial. 2008;21(2):150–4. [DOI] [PubMed] [Google Scholar]
- 11. Gou BD, Bian S, Zhang TL, Wang K.. Gadolinium-promoted precipitation of calcium phosphate is associated with profibrotic activation of RAW 264.7 macrophages. Toxicol In Vitro. 2010;24(6):1743–9. [DOI] [PubMed] [Google Scholar]
- 12. Del Galdo F, Wermuth PJ, Addya S, et al. . NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent omniscan: Possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69(11):2024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


