Figure 4.
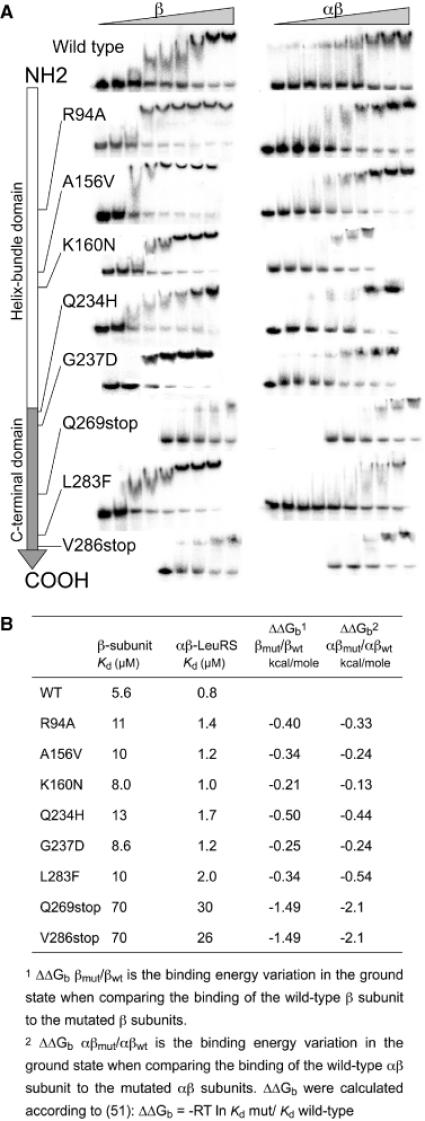
Measurement of the dissociation constant of LeuRS proteins for tRNALeu by gel-shift assay. (A) In vitro transcribed 32P-labelled A.aeolicus 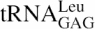 was incubated with increasing concentrations of the β subunits or whole αβ-LeuRS (see Materials and Methods). Apparent dissociation constants (Kd) of the proteins were estimated from quantification of the free and retarded tRNA. Band shifts are displayed along the linear schematic representation of the β subunit (NH2- to the COOH-end). For clarity the protein concentrations were omitted from each picture. The large increases of Kd were shown by a shift of the gel picture towards the right side, which symbolizes the high values. (B) Kd values and binding energy variations for the β subunits and αβ-LeuRS proteins.
was incubated with increasing concentrations of the β subunits or whole αβ-LeuRS (see Materials and Methods). Apparent dissociation constants (Kd) of the proteins were estimated from quantification of the free and retarded tRNA. Band shifts are displayed along the linear schematic representation of the β subunit (NH2- to the COOH-end). For clarity the protein concentrations were omitted from each picture. The large increases of Kd were shown by a shift of the gel picture towards the right side, which symbolizes the high values. (B) Kd values and binding energy variations for the β subunits and αβ-LeuRS proteins.
