Abstract
Background:
The first methadone maintenance treatment clinic in Tanzania was launched in February 2011 to address an emerging HIV epidemic among people who inject drugs. We conducted a retrospective cohort study to understand factors associated with linkage to HIV care and explore how a methadone maintenance treatment clinic can serve as a platform for integrated HIV care and treatment.
Methods:
This study used routine programmatic and clinical data on clients enrolled in methadone at Muhimbili National Hospital from February 2011 to January 2013. Multivariable proportional hazards regression model was used to examine time to initial CD4 count.
Results:
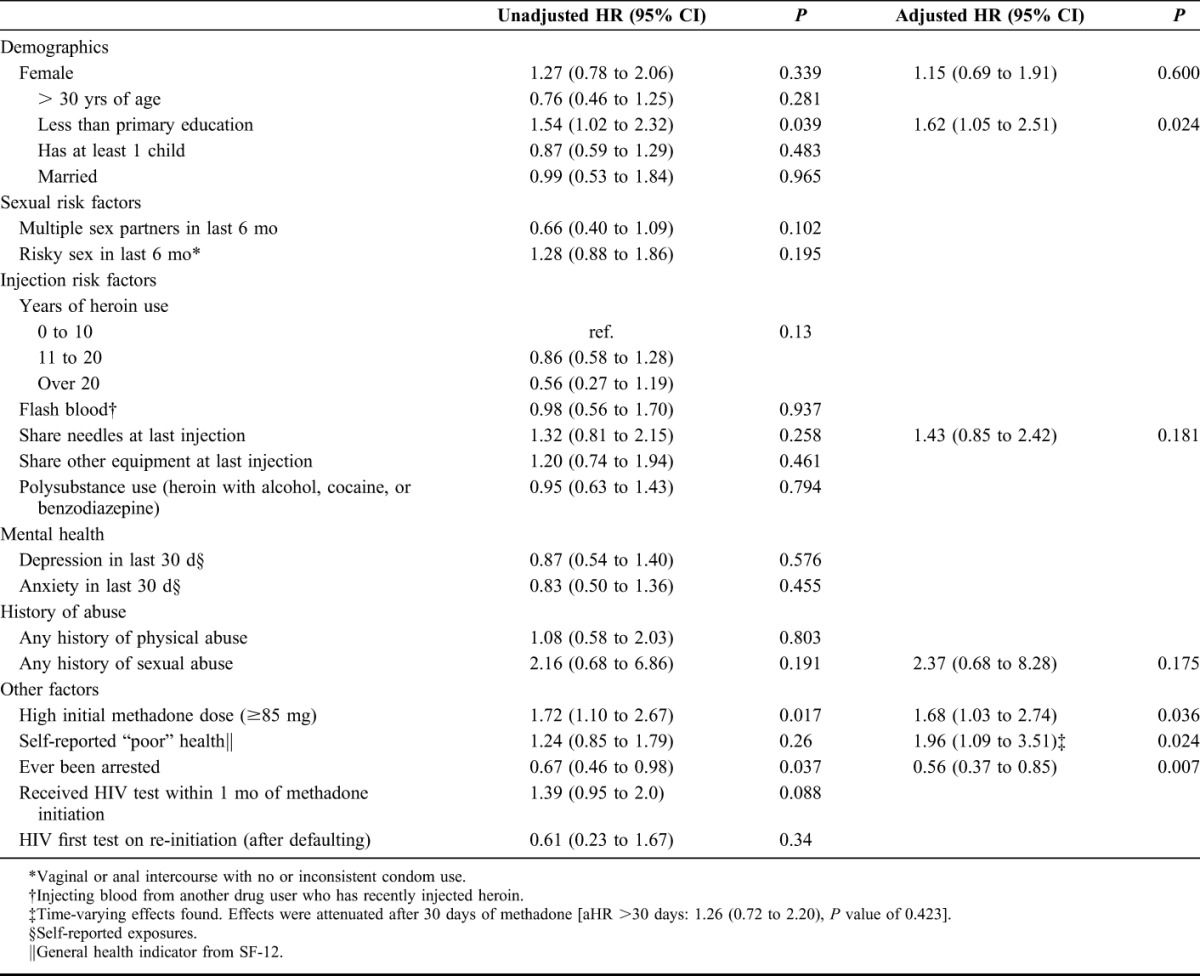
Final analyses included 148 HIV-positive clients, contributing 31.7 person-years. At 30, 60, and 90 days, the probability of CD4 screening was 40% [95% confidence interval (CI): 32% to 48%], 55% (95% CI: 47% to 63%), and 63% (95% CI: 55% to 71%), respectively. Clients receiving high methadone doses (≥85 mg/d) [adjusted hazard ratio (aHR): 1.68, 95% CI: 1.03 to 2.74] had higher likelihood of CD4 screening than those receiving low doses (<85 mg/d). Clients with primary education or lower (aHR: 1.62, 95% CI: 1.05 to 2.51) and self-reported poor health (aHR: 1.96, 95% CI: 1.09 to 3.51) were also more likely to obtain CD4 counts. Clients with criminal arrest history (aHR: 0.56, 95% CI: 0.37 to 0.85]) were less likely to be linked to care. Among 17 antiretroviral therapy eligible clients (CD4 ≤ 200), 12 (71%) initiated treatment, of which 7 (41%) initiated within 90 days.
Conclusions:
Levels of CD4 screening and antiretroviral therapy initiation were similar to Sub-Saharan programs caring primarily for people who do not inject drugs. Adequate methadone dosing is important in retaining clients to maximize HIV treatment benefits and allow for successful linkage to services.
Keywords: HIV, linkage to care, methadone, people who inject drugs, Sub-Saharan Africa, CD4
INTRODUCTION
Drug trafficking through East Africa emerged in the mid-1980s and continues to increase.1 Seizures of heroin in Africa, especially in East Africa, have increased 10-fold since 2009.1 As of 2011, an estimated 533,000 opiate consumers live in East Africa with an estimated 50,000 people who inject drugs (PWID) in Tanzania.2 In Dar es Salaam, Tanzania, PWID have an estimated HIV prevalence of 42%–51%, compared with 6.9% among the general population in the city.3,4 Furthermore, high HIV prevalence is accompanied by high burdens of hepatitis C (57%–76%) and active pulmonary tuberculosis (4%).5–7
Methadone maintenance treatment (MMT), HIV testing and counseling, and antiretroviral therapy (ART) are considered essential components of the comprehensive package of HIV prevention interventions for PWID, endorsed by the US Centers for Disease Control and Prevention, World Health Organization, United Nations Office on Drug and Crime, and Joint United Nations Programme on HIV/AIDS.8 Methadone is an effective treatment for opioid dependence, associated with lowering morbidity and mortality and reducing sexual and injection-related risk behaviors associated with drug use.9–14 Retention in MMT has led to optimized HIV prevention and treatment benefits, including routine testing and counseling and linkage to care and treatment for individuals living with HIV.9,15–17 As such, methadone is designated as an essential medicine by the World Health Organization.18
Studies from Sub-Saharan Africa estimate 59%–72% of individuals diagnosed with HIV ever obtain CD4 screening.19,20 In 2012, 59% of ART-eligible individuals in Tanzania, based on outdated CD4 criteria (<200), were currently on treatment.21 Despite scale-up of ART for people living with HIV, up to 26% of care and treatment clients become lost-to-follow-up.21 Compared with the general population, PWID face additional barriers, including delays in and denial of the provision of health care services, harassment by law enforcement, and fear of criminalization and stigmatization that impact access to and uptake of medical services in traditional settings.22–26 Because methadone clinics are designed to address the clinical needs of PWID, they can offer a unique venue for the provision of a full range of HIV-related and other health services.
We conducted a retrospective cohort study to understand the factors associated with linkage to HIV care and explore how an MMT clinic can serve as a platform for integrated HIV care and treatment. We hypothesize that enrollment in MMT with higher dosing is associated with timely linkage to HIV care.
METHODS
Study Setting
The first publicly funded MMT clinic in Tanzania was launched in February 2011, offering daily directly observed methadone at Muhimbili National Hospital (MNH) in Dar es Salaam. As part of routine care, clients were offered voluntary HIV testing and counseling on enrollment and at routine follow-up periods. For clients who tested HIV positive, the clinic performed blood draws and transported samples for CD4 screening to the central laboratory at MNH. Using traditional methods, the central laboratory conducted CD4 count testing and reported results back to the methadone clinic. MMT and clinical HIV services were available at MNH but not colocated. Therefore, clients were provided escorted in-person referrals to HIV clinical services at the care and treatment center (CTC), situated in a separate building on the hospital campus. For eligible individuals, the methadone clinic facilitated access to HIV therapy and provided daily codispensing of methadone and ART. Clients may also receive HIV screening through program caravans, which work in parallel with the methadone program to reach key populations in the surrounding district. Caravans provide mobile counseling and testing and referrals to HIV CTCs but do not provide methadone dosing or direct HIV care.
Study Population
Study subjects included MMT clients enrolled from February 2011 to January 2013 who tested positive for HIV after enrollment in methadone. Inclusion criteria for methadone initiation have been described previously.27 Additional criteria included (1) positive HIV test within 7 days before or any time after MMT initiation, (2) laboratory or clinical notes confirming HIV result, and (3) actively receiving MMT as of HIV-positive test date. We sought to capture individuals who tested positive for HIV through MMT-based services and were not previously linked to care. Therefore, individuals who indicated an approximate date of first HIV positivity before enrollment or received HIV care elsewhere were excluded from analysis.
Data Sources
This study used de-identified routine clinical and program monitoring data from the MMT clinic, which were extracted from the electronic databases. As part of routine care, health care providers and social workers conducted an in-person baseline assessment to collect demographic, health history, addiction severity,28 mental health,29 and HIV risk behavior data. Linkage to care data, including HIV testing and CD4 result dates, were obtained through medical chart review and abstraction. Daily methadone dosing data for each client were collected by the pharmacy.
Measures
Exposures
Our primary exposure of interest was methadone dose. The mean daily methadone dose during the first 3 months of treatment was calculated and categorized into low (<85 mg/d) or high (≥85 mg/d). Categorization of dose was based on previous research with this cohort and other literature regarding methadone dosing.30–33 Additional exposures of interest included demographics, sexual-, and injection-related risk factors, mental health, history of physical or sexual abuse, and arrest history.
Outcomes
Linkage to care for individuals testing HIV positive was defined as obtaining a CD4 count. The number of days between the first HIV-positive test and CD4 result was the primary outcome of interest. Follow-up time began at the HIV-positive result date (t0) and ended with the date of CD4 result, the last methadone dose for those experiencing a censoring event [death, involuntary discharge, medical withdrawal, or defaulting (ie, missed 21 consecutive doses)] or January 31, 2013, whichever occurred first. If a client tested positive through program caravans within 1 week of MMT enrollment, the initiation date was used as t0.
Statistical Methods
Multivariable Cox proportional hazards regression models were used to assess the relationship of exposures with obtaining a CD4 count among clients living with HIV. Backward stepwise regression with a criterion P value of 0.2 was used to select the variables for the multivariable model. Forward stepwise regression was used in sensitivity analysis to confirm variables. Cumulative incidence of CD4 testing, taking into account the competing risk of censoring events, was plotted by methadone dose. Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated to compare outcomes between groups. The proportional hazards assumption was tested using the Schoenfeld residuals. Self-reported poor health was found to have time-varying effects with linkage to care and was therefore specified in the model to estimate associations in early follow-up (≤30 days) and later follow-up (>30 days). Statistical analyses were conducted using Stata 12.1 (College Station, TX).
Ethics Statement
The use and analysis of de-identified programmatic data were approved by the US Centers for Disease Control and Prevention, E&I Review Services in the United States, and the ethical review committees at Muhimbili University of Health and Allied Sciences and the National Institute of Medical Research in Tanzania as program evaluation and nonhuman subjects research.
RESULTS
MMT Clients
During the study period, 629 individuals initiated MMT. Average age at enrollment was 32 (SD: ±6) years and 93% of clients were men. Among 469 (75%) clients who tested for HIV, 185 (39%) were confirmed HIV positive. Excluded cases included 21 (11%) that were missing test dates and 16 (9%) that tested positive outside the inclusion window. The final analysis included 148 HIV-positive MMT clients, contributing 31.7 person-years of follow-up. Table 1 includes baseline characteristics of methadone clients living with HIV, disaggregated by methadone dose.
TABLE 1.
Baseline Characteristics of Methadone Clients Living With HIV in Dar es Salaam, Tanzania (N = 148)

Linkage to Care
At the end of the study, 119 (80%) clients received at least 1 CD4 count and were active methadone clients, 14 (9%) were active clients but had not received screening, 14 (9%) defaulted from MMT before screening, and 1 (1%) was deceased. At 30, 60, and 90 days, the probability of clients undergoing CD4 screening was 40% (95% CI: 32% to 48%), 55% (95% CI: 47% to 63%), and 63% (95% CI: 55% to 71%), respectively.
Factors Associated With Linkage to Care
Figure 1 illustrates the cumulative incidence of CD4 screening by methadone dose. Table 2 includes adjusted and unadjusted HRs for the associations of patient characteristics and linkage to CD4 count among clients living with HIV. In the multivariable model, clients receiving ≥85 mg methadone/d [adjusted hazard ratio (aHR): 1.68, 95% CI: 1.03 to 2.74] had higher likelihood of CD4 screening than those receiving <85 mg methadone/d. In addition, clients with primary education or lower (aHR: 1.62, 95% CI: 1.05 to 2.51) and self-reported poor health [aHR (≤30 days): 1.96, 95% CI: 1.09 to 3.51] were more likely to obtain CD4 counts. Self-reported poor health was found to have time-varying effects that attenuated after the first 30 days of methadone treatment [aHR (>30 days): 1.26, 95% CI: 0.72 to 2.20]. Compared with clients with no arrest history, clients with a history of arrest (aHR: 0.56, 95% CI: 0.37 to 0.85) were less likely to obtain a CD4 count. Results from the sensitivity analysis showed qualitatively similar results.
FIGURE 1.
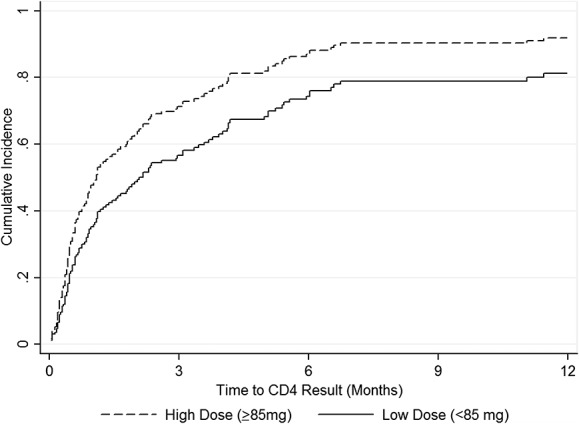
Cumulative incidence of initial CD4 screening by methadone dose.
TABLE 2.
Unadjusted and Adjusted Hazard Ratios for Linkage to CD4 Count Among Methadone Clients Living With HIV in Dar es Salaam, Tanzania (N = 148)
ART Eligibility and Initiation
Median initial CD4 count was 458 cells per microliter (interquartile range: 296–673) and 17 clients had CD4 ≤ 200, which served as the criterion for ART eligibility during our study period. Among ART-eligible clients, 12 (71%) initiated treatment, of which 7 (41%) initiated within 90 days of their CD4 result.
DISCUSSION
This first report on linkage to HIV care among methadone clients in Tanzania indicates similar levels of CD4 screening and ART initiation when compared with estimates from programs caring for non-PWID throughout Sub-Saharan Africa.19,20 We view this as a positive outcome because PWID, in general, tend to be at higher risk of being lost-to-follow-up than the general population living with HIV.34
This study expands on our previous implementation science research, focused on identifying gaps and developing strategies for improved HIV service delivery for drug users in Tanzania.27,30,35 Previous research indicated that the proportion of MNH clients retained in methadone at 12 months was 57% and that clients receiving higher methadone doses had a lower risk of attrition.30,36–38 Engagement and retention in MMT is critical because 82% of PWID who drop out return to injection within 10–12 months.14 In addition, the stability provided to clients through methadone treatment is essential for successful linkage to other health services. These results support a growing body of the literature that engagement in MMT can lead to optimized HIV prevention and treatment benefits.9,13,15–17 Our data indicated that stabilization through higher methadone doses increased the probability of obtaining a CD4 count. Our results also showed stronger effects of self-perceived poor health, a component of health-related quality of life, on linkage in the first 30 days of methadone treatment. In contrast, individuals with a history of arrest were less likely to be linked to care, highlighting the need for enhanced case management. Successful implementation approaches should incorporate appropriate adequate dosing strategies and concurrently address individual level factors to keep clients engaged in care.
Enhancing HIV prevention, care, and treatment services for PWID and other key populations is critical to the global response to HIV. Innovative evidence-based strategies that have shown effectiveness in traditional settings should be adapted and operationalized in settings that serve hard-to-reach populations. In particular, it is important to consider client-centered integrated strategies that link and retain clients into needed services.35,39
Although 80% of clients were linked to care during the study period, reductions in the amount of time to linkage are needed. In some cases, CD4 testing took several months. The methadone program, which currently requires daily attendance, can provide a platform for regular HIV testing and immediate linkage to care for those who test positive. However, current CD4 testing technology burdens systems and clients as specimens are drawn at the clinic and transported to the central hospital laboratory. Reagent shortages may require transport of samples to an off-site laboratory, further delaying the CD4 testing process. The use of point-of-care technologies in HIV clinics has improved clinical monitoring and streamlined ART delivery in other settings.40–43 In addition, field studies demonstrate feasibility and technical validity in program settings in Africa.41–43 Integration of point-of-care CD4 instruments in the methadone clinic could allow clients to receive both HIV and CD4 test results in a single visit, thus potentially closing the loss-to-care gap.
As a critical next step in the continuum of care, improvements in linkage to HIV treatment are needed. During the study period, the clinic provided escorted referrals to the off-site CTC and worked to facilitate access to ART. However, many factors, some of which can be complicated by drug use, contribute to timely ART initiation. Although not the focus of this article, examination of systemic, structural, and individual level barriers to ART initiation will be important to fully characterize the HIV treatment cascade among our client population and inform the development of new approaches.
Strengths of the study included standardized clinical protocols, client tracing, and data recording. However, clinical HIV data relied on accurate chart abstraction and timely transfer of information from the central hospital laboratory and CTC, located outside the methadone clinic. The primary limitation is the observational nature of our research. As a result, the potential for unmeasured or mismeasured factors to bias our results existed. Clients who defaulted from methadone were not systematically followed up to assess CD4 screening in other locations. In this study, we assumed that these individuals did not receive HIV-related services elsewhere, given the levels of stigma and refusal of services that PWID commonly face. Future analyses will examine how attrition from and re-entry into the methadone program affect continuity of HIV testing and linkage to care. Additionally, the nature of our data limited our capacity to evaluate the subprocesses of the care continuum, including time between blood draw, laboratory testing, and CD4 count result. We were unable to evaluate the proportion of clients receiving CD4 results. However, we assume that given the structure of care, a high proportion of clients return for their CD4 results during a follow-up visit. As the program expands to integrate additional HIV services, a strengthened monitoring and evaluation system will be critical to documenting and improving the care pathway.
As programs expand in Tanzania and initiate in other African countries, identifying and implementing strategies that engage clients are critical to further linking PWID into needed health services. Our research supports the use of methadone programs as a gateway to HIV prevention, treatment, and care for PWID and as platforms for integrated health services in resource-constrained settings. In addition, our results highlight the importance of higher doses of methadone to maximize HIV treatment benefits for PWID. Future initiatives will focus on developing an integrated platform of MMT and HIV treatment delivery to improve linkage to care and ART initiation.
ACKNOWLEDGMENTS
The authors would like to thank the clients, providers, staff and partners of the methadone clinic at Muhimbili National Hospital.
Footnotes
Supported by the National Institutes of Health, National Institute on Drug Abuse (Grant No. 1R34DA037787).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.UNODC. World Drug Report 2013. Vienna, Austria: Available at: http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf. Accessed January 2, 2014. [Google Scholar]
- 2.UNODC. The Global Afghan Opium Trade: A Threat Assessment. 2011. Available at: http://www.unodc.org/documents/data-and-analysis/Studies/Global_Afghan_Opium_Trade_2011-web.pdf. Accessed January 2, 2014. [Google Scholar]
- 3.Williams ML, McCurdy SA, Bowen AM, et al. HIV seroprevalence in a sample of Tanzanian intravenous drug users. AIDS Educ Prev. 2009;21:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanzania Commission for AIDS, Zanzibar AIDS Commission, National Bureau of Statistics, Office of Chief Government Statistician Zanzibar, ICF International. Third Tanzania HIV/AIDS and Malaria Indicator Survey 2011-2012. Available at: http://dhsprogram.com/pubs/pdf/AIS11/AIS11.pdf. Accessed December 10, 2014. [Google Scholar]
- 5.Nyandindi C, Mbwambo J, McCurdy S, Lambdin B, Copenhaver M, Bruce RD. Prevalence of HIV, hepatitis C and depression among people who inject drugs in the Kinondoni Municipality in Dar es Salaam, Tanzania. 75th Annual Meeting - College on Problems of Drug Dependence. San Diego. June 15 - 20, 2013. Abstract 473/Poster 113.
- 6.Chang O, Bruce RD, Masao F, et al. Risk factors associated with HCV infection and prevalence of HIV-HCV co-infection among people who inject drugs receiving medication-assisted treatment in Dar es Salaam, Tanzania. Paper presented at: 20th International AIDS Conference; July 24, 2014. Abstract THAC0405. Melbourne, Australia.
- 7.Gupta A, Mbwambo J, Mteza I, et al. Active case finding for tuberculosis among people who inject drugs on methadone treatment in Dar es Salaam, Tanzania. Int J Tuberc Lung Dis. 2014;18:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.PEPFAR. Comprehensive HIV Prevention for People Who Inject Drugs, Revised Guidance. Available at: http://www.pepfar.gov/documents/organization/144970.pdf. Accessed January 4, 2014. [Google Scholar]
- 9.Bruce RD. Methadone as HIV prevention: high volume methadone sites to decrease HIV incidence rates in resource limited settings. Int J Drug Policy. 2010;21:122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger DS, Woody GE, McLellan AT, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–1056. [PubMed] [Google Scholar]
- 11.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171, iii-iv. [DOI] [PubMed] [Google Scholar]
- 12.Gibson DR, Flynn NM, McCarthy JJ. Effectiveness of methadone treatment in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 1999;13:1807–1818. [DOI] [PubMed] [Google Scholar]
- 13.Woody GE, Bruce D, Korthuis PT, et al. HIV risk reduction with buprenorphine-naloxone or methadone: findings from a randomized trial. J Acquir Immune Defic Syndr. 2014;66:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment: Patients, Programs, Services and Outcome. Vol 14. New York, NY: Springer-Verlag Publishing; 1991. [Google Scholar]
- 15.Uhlmann S, Milloy MJ, Kerr T, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood E, Hogg RS, Kerr T, et al. Impact of accessing methadone on the time to initiating HIV treatment among antiretroviral-naive HIV-infected injection drug users. AIDS. 2005;19:837–839. [DOI] [PubMed] [Google Scholar]
- 17.Knowlton A, Hoover DR, S-e Chung, et al. Access to medical care and service utilization among injection drug users with HIV/AIDS. Drug and Alcohol Dependence. 2001;64:55–62. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO Model List of Essential Medicines: 17th List. Available at: http://www.who.int/medicines/publications/essentialmedicines/en/. Accessed December 8, 2014. [Google Scholar]
- 19.Rosen S, Matthew P. Retention in HIV care between testing and treatment in sub-Saharan africa: a systematic review. PLoS Med. 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mugglin C, Estill J, Wandeler G, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17:1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PEPFAR. Tanzania Operational Plan Report: FY 2013. Available at: http://www.pepfar.gov/documents/organization/222184.pdf. Accessed December 10, 2014. [Google Scholar]
- 22.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. [DOI] [PubMed] [Google Scholar]
- 23.Mimiaga MJ, Safren SA, Dvoryak S, et al. “We fear the police, and the police fear us”: structural and individual barriers and facilitators to HIV medication adherence among injection drug users in Kiev, Ukraine. AIDS Care. 2010;22:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood E, Kerr T, Tyndall MW, et al. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–1256. [DOI] [PubMed] [Google Scholar]
- 25.Paxton S, Gonzales G, Uppakaew K, et al. AIDS-related discrimination in Asia. AIDS Care. 2005;17:413–424. [DOI] [PubMed] [Google Scholar]
- 26.Ding L, Landon BE, Wilson IB, et al. Predictors and consequences of negative physician attitudes toward HIV-infected injection drug users. Arch Intern Med. 2005;165:618–623. [DOI] [PubMed] [Google Scholar]
- 27.Lambdin BH, Bruce RD, Chang O, et al. Identifying programmatic gaps: inequities in harm reduction service utilization among male and female drug users in Dar es Salaam, Tanzania. PloS one. 2013;8:e67062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7:79–110. [DOI] [PubMed] [Google Scholar]
- 30.Lambdin BH, Masao F, Chang O, et al. Methadone treatment for HIV prevention-feasibility, retention, and predictors of attrition in Dar es Salaam, Tanzania: a retrospective cohort study. Clin Infect Dis. 2014;59(5):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fareed A, Casarella J, Amar R, et al. Methadone maintenance dosing guideline for opioid dependence, a literature review. J Addict Dis. 2010;29:1–14. [DOI] [PubMed] [Google Scholar]
- 32.Brady TM, Salvucci S, Sverdlov LS, et al. Methadone dosage and retention: an examination of the 60 mg/day threshold. J Addict Dis. 2005;24:23–47. [DOI] [PubMed] [Google Scholar]
- 33.Mattick RP, Kimber J, Breen C, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:Cd002207. [DOI] [PubMed] [Google Scholar]
- 34.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. [DOI] [PubMed] [Google Scholar]
- 35.Bruce RD, Lambdin B, Chang O, et al. Lessons from Tanzania on the integration of HIV and tuberculosis treatments into methadone assisted treatment. Int J Drug Policy. 2014;25:22–25. [DOI] [PubMed] [Google Scholar]
- 36.Sarasvita R, Tonkin A, Utomo B, et al. Predictive factors for treatment retention in methadone programs in Indonesia. J Subst Abuse Treat. 2012;42:239–246. [DOI] [PubMed] [Google Scholar]
- 37.Bao YP, Liu ZM, Epstein DH, et al. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang PW, Wu HC, Yen CN, et al. Change in quality of life and its predictors in heroin users receiving methadone maintenance treatment in Taiwan: an 18-month follow-up study. Am J Drug Alcohol Abuse. 2012;38:213–219. [DOI] [PubMed] [Google Scholar]
- 39.Sylla L, Bruce RD, Kamarulzaman A, et al. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynberg E, Cooke G, Shroufi A, et al. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–1579. [DOI] [PubMed] [Google Scholar]
- 42.Larson BA, Schnippel K, Ndibongo B, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61:e13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mtapuri-Zinyowera S, Chideme M, Mangwanya D, et al. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr. 2010;55:1–7. [DOI] [PubMed] [Google Scholar]


